Brief

The pain is becoming all too common for many pharmaceutical executives. After a decade or more of investment in drug development and clinical trials, a company launches a promising new product only to see sales fall far short of expectations. Our research shows that nearly 50% of launches over the past eight years have underperformed analyst expectations, and more than 25% have failed to reach even 50% of external revenue forecasts.
That’s a serious concern for the pharma industry, which is expected to derive 25% to 80% of its revenue from new launches by 2021. While the level of clinical differentiation plays an important role in a drug’s success, there are many examples of successful drugs that were not considered a major clinical breakthrough at launch, such as Bayer’s and Johnson & Johnson’s Xarelto. To understand how success factors that are within the control of a launch team are changing, we surveyed 100 senior launch executives from the top 20 pharmaceutical companies. The Bain & Company study examined the performance of each launch across 20 launch activities.
Our findings show that companies with successful launches do three things right:
- They differentiate their drug through messaging, post-launch data and services.
- They create broad customer advocacy via a superior customer experience.
- They organize their launch as a micro-battle and ensure continuous frontline feedback.
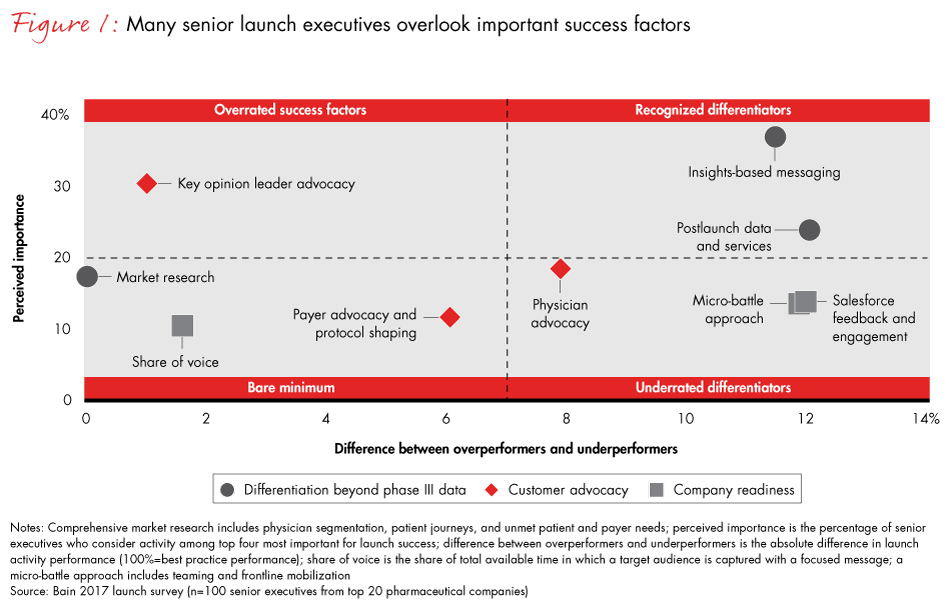
Other factors, such as comprehensive market research, key opinion leader advocacy and competitive resourcing—all important for a successful launch—do not ensure outperformance (see Figure 1). Our research also shows that executives significantly underestimate several key success factors, including customer advocacy and organizing the launch as a micro-battle. And while they understand the importance of messaging, post-launch data and services, they often fail to effectively communicate their market research to payers and providers.
Go beyond clinical trial results to make your product stand out
New drug launches face more intense competition today than they faced a decade ago. The average window of time in which a drug remains on the market before competing products arrive has fallen to four years, down from eight years between 2000 and 2004. The onslaught of new products makes it much harder to use phase III clinical trial data alone to differentiate a drug in the eyes of doctors, regulators and health insurers.
Companies with launches that repeatedly outperform expectations are adept at communicating the key clinical and nonclinical benefits of a new product to prescribing physicians and other decision makers. Often they also use post-launch data and services to further differentiate their product from the competition.
Most senior executives understand that effective marketing messaging is critical to launch success. But getting it right is not easy. Our survey and experience show a large gap between winners and losers.
Companies with the most effective messaging follow three key guidelines. First, they translate research into actionable insights. All the companies we surveyed said they use market research tools, including patient pathways, physician segmentation and focus groups. However, only those with successful launches transformed market data into actionable insights to make their product stand out. These messages are grounded in clinical data and built on efficacy and safety, but they also take into consideration the cognitive shortcuts doctors deploy when they learn about new treatments and make decisions (heuristics). Understanding these pathways can help pharma companies communicate the benefits of their medicines more effectively.
Bayer’s successful launch of its anticoagulant drug Xarelto highlights the power of effective messaging. Even though Xarelto was second to market and many stakeholders did not consider it the most effective compound in its class at the time of launch, it rapidly became a market leader. Xarelto used simplicity as a differentiation factor: It offered once-a-day dosing compared with twice-daily for Boehringer Ingelheim’s first-to-market rival drug Pradaxa.
A second important success factor is conducting post-launch studies to close any remaining gaps in data and ensure superior data quality over competing products and new entrants. High-quality data, in turn, enhances market access. Successful companies put a post-launch evidence-generation plan in place 18 months before the launch to generate a steady stream of data after the launch that supports the drug’s efficacy. Take the example of Celgene, which produced almost twice as many post-launch studies in Europe for its multiple myeloma drug Revlimid as compared with its closest competitor. Celgene’s steady drumbeat of fresh evidence has given Revlimid a competitive advantage by keeping it at the forefront of hematologists’ minds.
Third, successful companies increase the effectiveness of their messaging by including a competitive service offer to address patient and physician pain points—a factor many pharma executives overlook or undervalue. Physician services, such as diagnostics, patient identification, onboarding, reimbursement support and compliance, play a significant role in successful launches. During the recent launch of a neurology drug by a top pharma company, for example, the launch team noted that the administrative burden of getting patients started on therapy was a crucial pain point. In response, the team invested far beyond the initial plan to relieve the burden.
Rafael Natanek, a partner with Bain's Healthcare practice, discusses three things that contribute to the success of a drug launch.
Build customer advocacy through superior customer experience
Physicians today consider a much wider set of factors beyond clinical data when deciding which drug to prescribe, including clinical protocols, drug price, the type of patient to whom a drug can be prescribed and overall treatment regimen. And they are rapidly shifting to a broader array of information sources, especially online sites and peers.
Our research shows that at least 40% of physicians’ brand preference is attributable to customer experience factors beyond the product, including, for example, how well pharma companies support physicians by providing answers to medical questions, identifying patients and connecting physicians with peers.
Many pharma companies, however, focus their advocacy activities on the most influential physicians in a given field—the key opinion leaders—and miss the opportunity to create advocates among the day-to-day prescribers. Bain research shows physicians give pharma companies an average Net Promoter Score® of negative 11% across all their interactions with them. That negative score highlights the significant potential to increase launch success by designing and delivering a superior customer experience to physicians.
Leading companies look beyond the key opinion leaders and turn day-to-day prescribers into advocates by providing them with a superior customer experience. They understand that a physician’s overall customer experience is a sum of individual interactions with a pharma company. Every interaction or episode is an opportunity to engage in a positive dialogue. One example is providing easy and accurate medical science liaison contacts within 24 hours of the physician’s request. Our study showed some of these interactions have a greater impact on prescribing behavior than others.
Organize your launch as a micro-battle
New drug launches today require greater coordination across the entire organization, including market access, patient services, medical affairs, regulatory, marketing and sales. Many pharma companies continue to rely on loosely organized cross-functional teams with a nominal global leader. The problem with that approach is that functional silos control the power, budget and talent decisions. And silo leaders manage drug launches via checklist, a rote approach that undercuts the group’s ability to raise or respond to critical issues and make cross-functional decisions.
Companies that outperform organize drug launches into micro-battles (see Figure 2). They create a company within the company, giving launch teams the authority and agility to make decisions that are best for the patient or the brand. Importantly, a micro-battle approach allows launch teams to focus on strategic issues for the success of the launch, not for checklists. Working with different scenarios instead of static market insights and a linear launch plan, they look out far beyond the launch date.
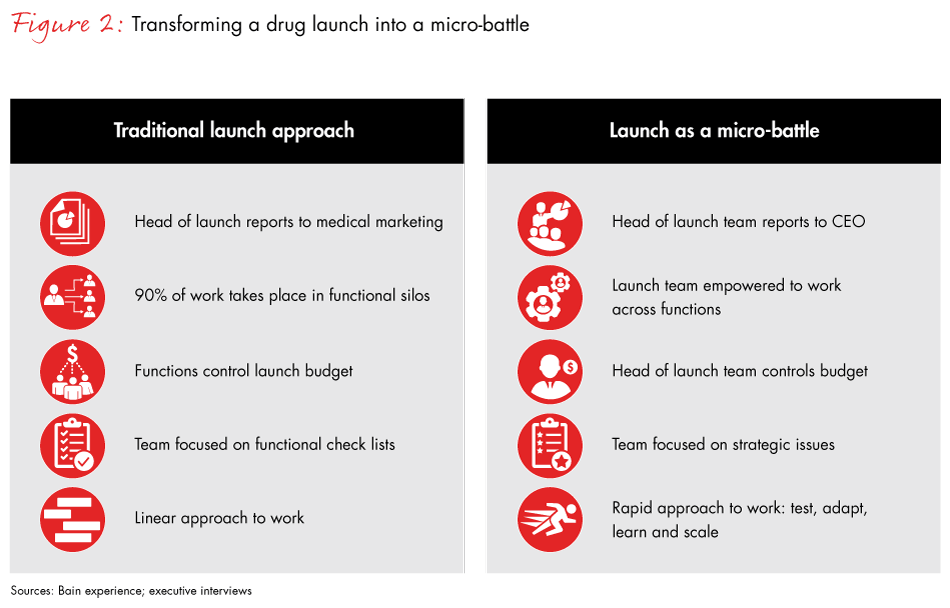
Pharma companies that approach the drug launch as a micro-battle move quicker, make bolder decisions and achieve superior launch results. They are more flexible and adapt rapidly to competitors, regulatory changes or new insights. Our data highlights this important shift: The most consistently undervalued factor contributing to a successful launch is the way leadership teams organize and manage the launch process.
How does a micro-battle strategy work? The first step is assembling the right cross-functional team—namely, 10 to 20 high-potential individuals who report to a launch CEO. The launch CEO is a senior executive who owns the micro-battle results and reports directly to the company CEO. These teams, which can include marketing and medical brand leaders as well as supply and commercial leaders from core countries, collect customer feedback before, during and after the launch, and make rapid adjustments as needed to the launch strategy. The launch CEO has decision rights or at least strong recommendation rights on strategy, resourcing and staffing decisions for the brand. Leadership teams support the micro-battle, affirming decisions, removing roadblocks, securing resourcing and coaching.
But my launch is different
Pharma companies that repeatedly outperform expectations for new product launches develop new sources of differentiation, focus on building superior customer experiences and treat drug launches as a micro-battle. Of course, where exactly a company invests within these three areas will differ, depending on clinical superiority and category leadership in a given field. An effective strategy is tailored to these two important factors.
Leadership teams interested in benchmarking their launch results against this powerful approach can start by posing the following questions:
- How did our last three launches perform vs. expectations, and what were the reasons for underperformance or overperformance?
- Have we identified all potential differentiation areas of our drug and incorporated them into our messaging?
- Which interactions matter most for our target physicians, and do we provide a superior customer experience?
- What are the three largest internal challenges the launch team faces, and what would it take to eliminate them?
Rafael Natanek is a partner with Bain & Company in the London office, Christoph Schlegel is a Bain partner in the Frankfurt office, Michael Retterath is a Bain partner in the New York office and George Eliades is a partner with Bain in the San Francisco office.
The authors wish to give special thanks to Gilbert Grima, Nathalie Fetzer-Hoernig, Anna Boleininger, Will Menz, Nicole Sinclair and Giovanni Miani for their contributions to this report. Additional thanks to Gail Edmondson for her editorial support.
Net Promoter®, Net Promoter System®, Net Promoter Score® and NPS® are registered trademarks of Bain & Company, Inc., Fred Reichheld and Satmetrix Systems, Inc.

