Article

Speed in clinical trials is non-negotiable—each day of delay in a clinical trial can reduce future sales by an estimated $500,000, per Tufts Center for the Study of Drug Development. Meanwhile, trials continue to become more complex, and competition is only increasing.
Written in collaboration with
Written in collaboration with

Biopharma companies aspire to make data-driven decisions around their clinical programs. To that end, they track many measures, including a broad set of internal and external financial and scientific considerations that affect R&D productivity. But one critical area remains relatively underanalyzed: operational performance, or how sponsors design and run timely, predictable clinical programs.
One challenge in judging operational performance is the lack of consolidated clinical intelligence. Traditional methods rely on slow, manual tasks—sifting through massive unstructured databases, consolidating findings, and relying on expert networks for added context.
AI is poised to change that. By integrating diverse data sources efficiently, AI can provide actionable clinical intelligence, answer specific queries, and uncover hidden patterns. Bain and AppliedXL have collaborated to create a new AI-powered approach that combines language models with human expertise to accelerate decision making and optimize clinical strategy.
Turning data into actionable insights
AppliedXL’s AI system continuously monitors a wide range of clinical trial events and performance metrics, organizing them into a readily queryable format. It captures risk signals and shifts across more than 100 categories, including irregular status progressions, timeline delays, and enrollment fluctuations.
With AI and public data, we can generate a real-time snapshot of a single trial, providing a comprehensive view of the overall progress and potential risks, such as those in Incyte’s ruxolitinib program for atopic dermatitis (AD) (see Figure 1).
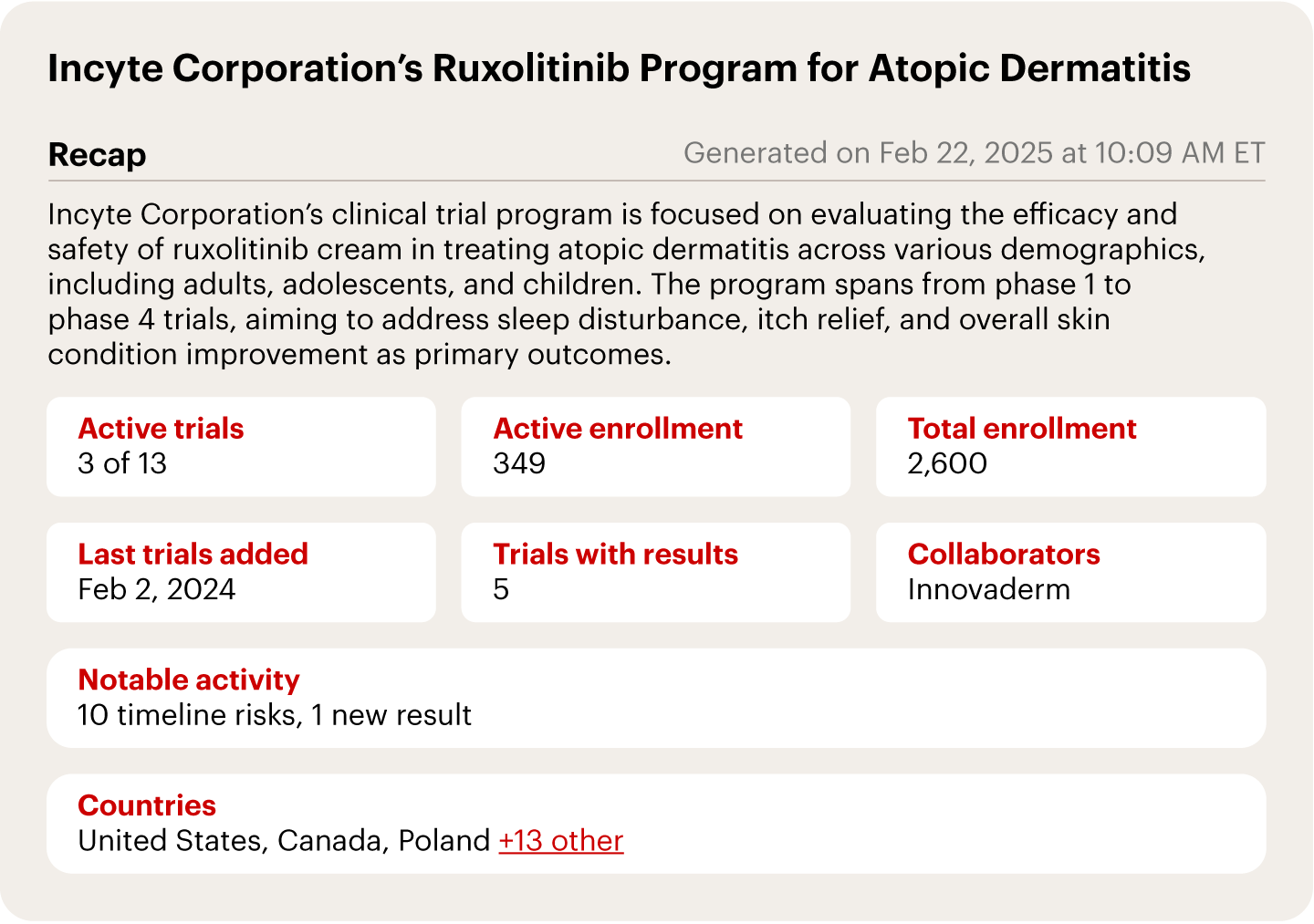

Notes: Clinical program data from analysis of Incyte Corporation’s public corporate and clinical filings; clinical program classifications are AI-inferred based on drug characteristics, trial design, and stated objectives
Sources: AppliedXL; Incyte Corporation public filingsAppliedXL’s risk detection has already demonstrated predictive power in identifying program stoppages or failures. For example, trials delayed by more than 150 days are 41% more likely to be terminated, according to a report from AppliedXL and Frank David, professor of the practice of biotechnology at Tufts University. Similarly, among trials that experienced a substantial decrease in enrollment, 53% were stopped early rather than following their expected trajectory to completion.
The benefits of this AI-powered approach are twofold:
- A better understanding of operational performance and risks by disease area. Sponsors can assess trial complexity to make investment decisions and design trials that anticipate unique roadblocks.
- Sharper measurement and benchmarking of clinical execution. Companies can analyze their trial delivery performance, compare against competitors, and identify opportunities for improvement.
Armed with AI insights, leading biopharma companies will streamline clinical development, cut costs, and speed up the journey from discovery to market.
Introducing PRIME: A smarter approach to trial execution
AI alone isn’t enough—human oversight is essential for high-stakes clinical decisions. To help organizations tap into AI’s power while keeping human judgement at the center, we developed PRIME, a five-step framework for clinical strategy.
P: Program identification
- AI action: Groups clinical trials into programs based on commonalities in drug characteristics, targeted indications, and trial objectives.
- Outcome: Concise program descriptions and rapid insight into research strategy, risks, and progress.
- Why AI? Traditionally, linking trials to specific programs has been a tedious, manual process.
R: Risk monitoring and competitor mapping
- AI action: Detects potential problems across nearly 100 event categories with real-time data monitoring.
- Outcome: The insights needed for timely, informed decision making on trial risks and the competitive landscape.
- Why AI? Managing the volume and complexity of clinical trial updates is laborious for analysts.
I: Identification of drug targets
- AI action: Analyzes diverse data sources to pinpoint drug targets, including molecules or pathways crucial in disease progression.
- Outcome: A dynamic repository for identifying novel targets, exploring drug repurposing opportunities, and understanding biological mechanisms.
- Why AI? Dispersed data sources and rapid scientific advancements make manual tracking costly and time consuming.
M: Market- and therapy-level analysis
- Human action: Interpret AI-generated insights at both the market and therapy levels to design and plan clinical trials.
- Outcome: Robust, data-backed clinical trial plans aligned with the identified programs and targets.
- Why humans? Analysts bring deep therapeutic area expertise, an understanding of clinical nuances, and the context of real-world clinical practice, ensuring trial designs are clinically relevant and strategically sound.
E: Execution strategy development
- Human action: Perform company comparisons and monitor clinical trial performance.
- Outcome: A comprehensive, adaptive, and optimized execution strategy.
- Why humans? Analysts provide critical insights into competitive positioning, strategic adjustments, stakeholder perspectives, and nuanced performance metrics.
AI in action: Risk monitoring and competitor mapping
To illustrate how AppliedXL and the PRIME approach work in practice, we analyzed trial execution across three disease areas: AD, non-small cell lung cancer (NSCLC), and heart failure (HF). We looked at all US-based trials across all phases—while removing outliers, such as those involving extreme enrollment shifts—to determine the percentage of trials delayed by disease area, the number of changes to timelines, the percentage of trials with an enrollment increase, and more.
What does the disease area data reveal? NSCLC trials experience the longest and highest share of trial delays, though AD trials see the most timeline changes. While the share of trials with enrollment increases is roughly consistent across NSCLC and AD, NSCLC shows almost three times the average increase in enrollment size (see Figure 2).
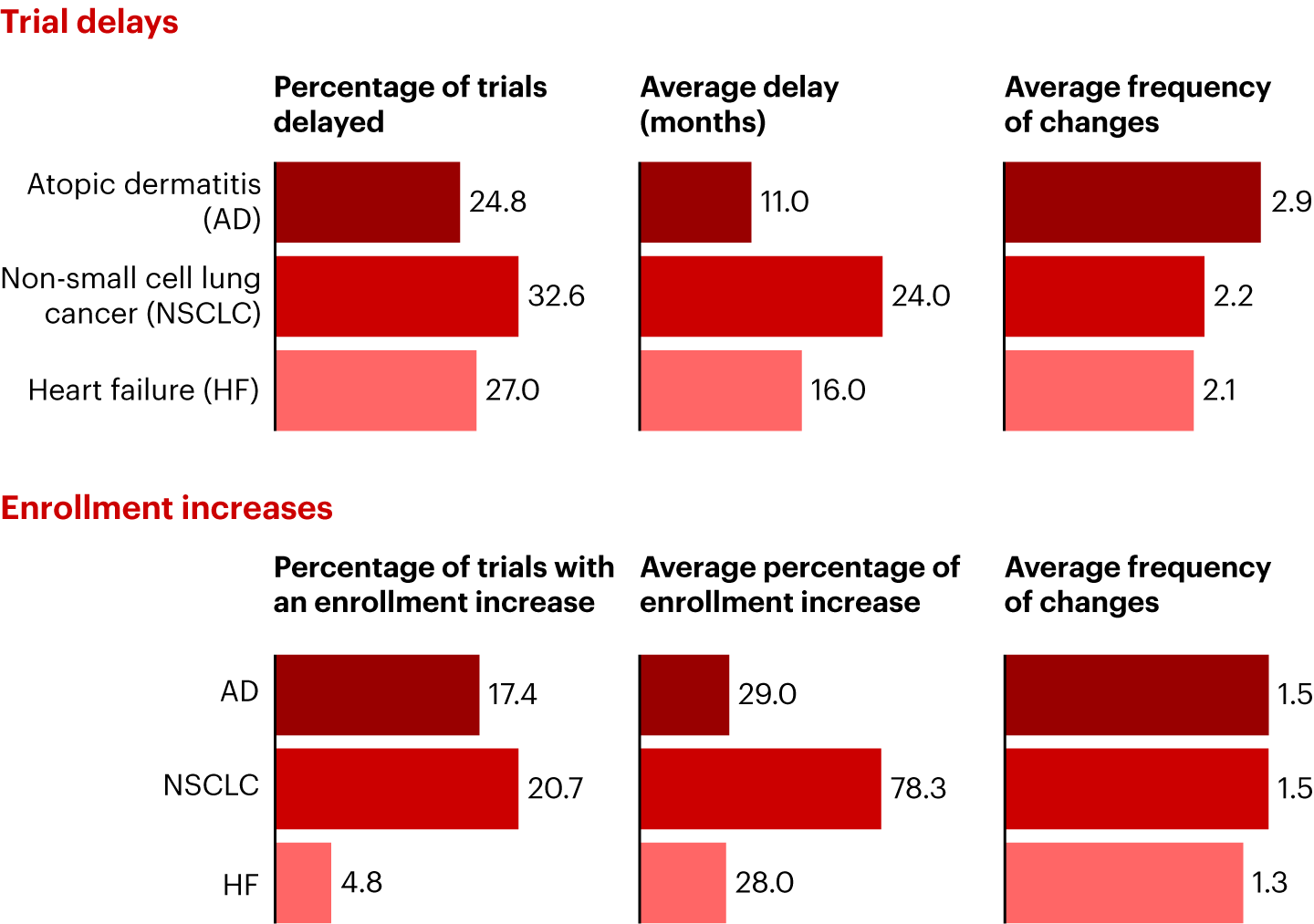

Notes: Percentage of trials delayed and with an enrollment increase is an average of top companies and a grouping of other peers; multiple NSCLC trials report an enrollment increase above 100%; NSCLC average percentage enrollment increase excludes one outlier trial that was canceled after enrolling only two patients
Sources: AppliedXL; Bain analysisInstead of an arduous manual analysis, AI helps us quickly reach a few conclusions. There’s only a slight difference in overall clinical trial execution and operation risk. The trends in delays and enrollment changes are likely a reflection of disease complexity and evolving competition for patients, as well as the rapidly evolving trial landscape. NSCLC’s diverse subtypes and molecular profiles could affect trial design, potentially requiring adjusted eligibility criteria.
Next, we aggregated measures by sponsor. By creating a total risk score to capture the aggregate frequency and magnitude of delays and enrollment changes, we found a notable difference in trial execution risk at the company level (see Figure 3).
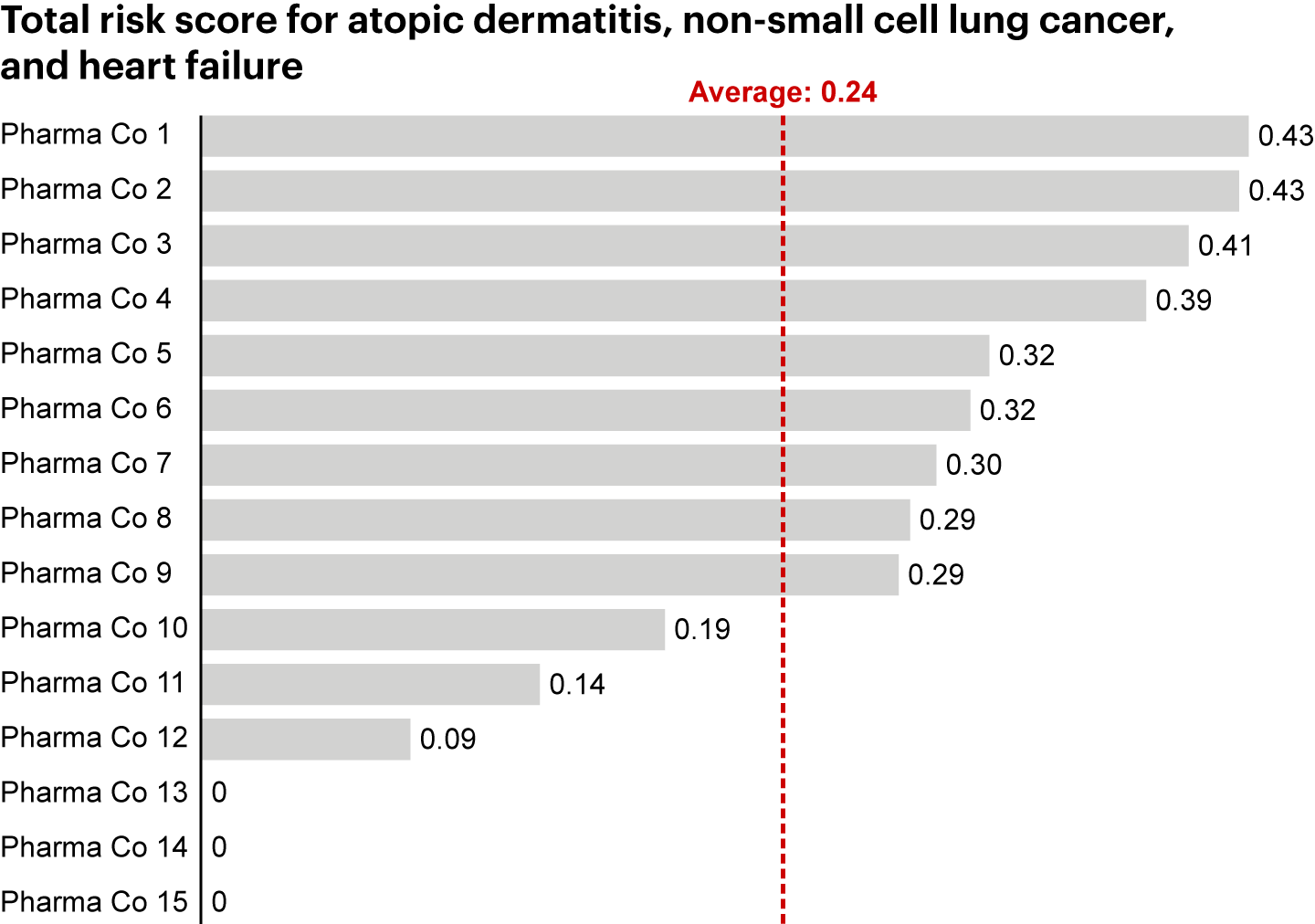

Notes: Accounts for US-only clinical trials conducted from 2014–2024; risk score is aggregate of percentage of delayed trials, average frequency and magnitude of change in delayed trials, percentage of trials with an enrollment increase, and average frequency and magnitude of change in trials with an enrollment increase
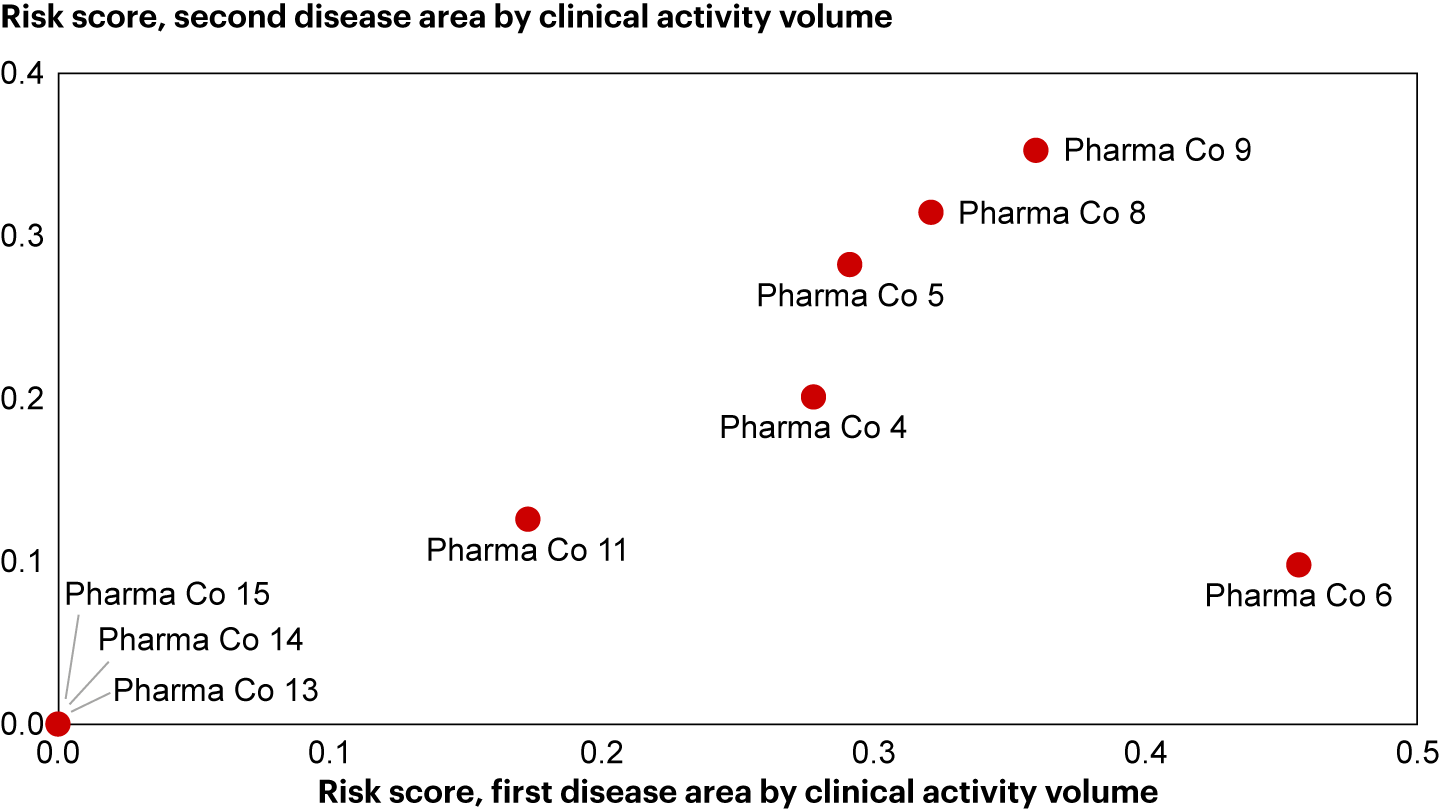
Sources: AppliedXL; Bain analysisWe also found that some companies have consistent risk scores across disease areas (see Figure 4). Therefore, the differences in performance between companies may be due to different “postures” toward trial planning and delivery. Some favor a “zero-defect” approach, planning conservatively and minimizing changes. Others embrace agility, moving fast and making changes as needed. By understanding their own posture and others’, firms can quickly take a pulse of the competitor landscape, compare their trial execution strategies, and refine their approach accordingly.

AI-powered insights, combined with human expertise, are redefining clinical trial strategy. With AppliedXL and the PRIME approach, we can help clinical development and operations leaders benchmark performance, improve trial design and planning, assess trial execution efficiency before entering new therapeutic areas, and make more confident investment decisions. In today’s high-stakes clinical landscape, speed and precision are everything—and AI is key to unlocking both.

About AppliedXL
AppliedXL is building the world’s first fully autonomous AI analyst, engineered to deliver real-time, research-grade insights that meet the standards of domain experts. Starting in the biopharma sector, AppliedXL equips decision makers with critical information—before it becomes news
