Report

Executive Summary
- Biopharma private equity buyouts remained strong, with the number of deals increasing markedly to 150 from 85 in 2019.
- However, disclosed deal values declined sharply to $21.4 billion from $40.7 billion in 2019, due to a lower number of deals valued at over $1 billion.
- Biopharma deal flow suffered less than other sectors from Covid-19, with investors targeting pharma services investments in North America and Europe. The Asia-Pacific region saw the most deals, including a large number of smaller, earlier-stage investments, often with direct molecule risk.
- Major investment themes included commercialization platforms, modernization of clinical trials, specialty pharma, and cell and gene therapy.
This article is part of Bain's 2021 Global Healthcare Private Equity and M&A Report.
After a banner 2019, disclosed biopharma deal value dropped sharply to $21.4 billion in 2020 from $40.7 billion, given a decline in very large deals and no megadeals like the Nestlé Skin Health $10.1 billion deal in 2019. Still, value was higher than all other years prior to 2019 (see Figure 1), and volume surged to 150 deals, up from 85 in 2019.
Disclosed deal value dropped from the 2019 high
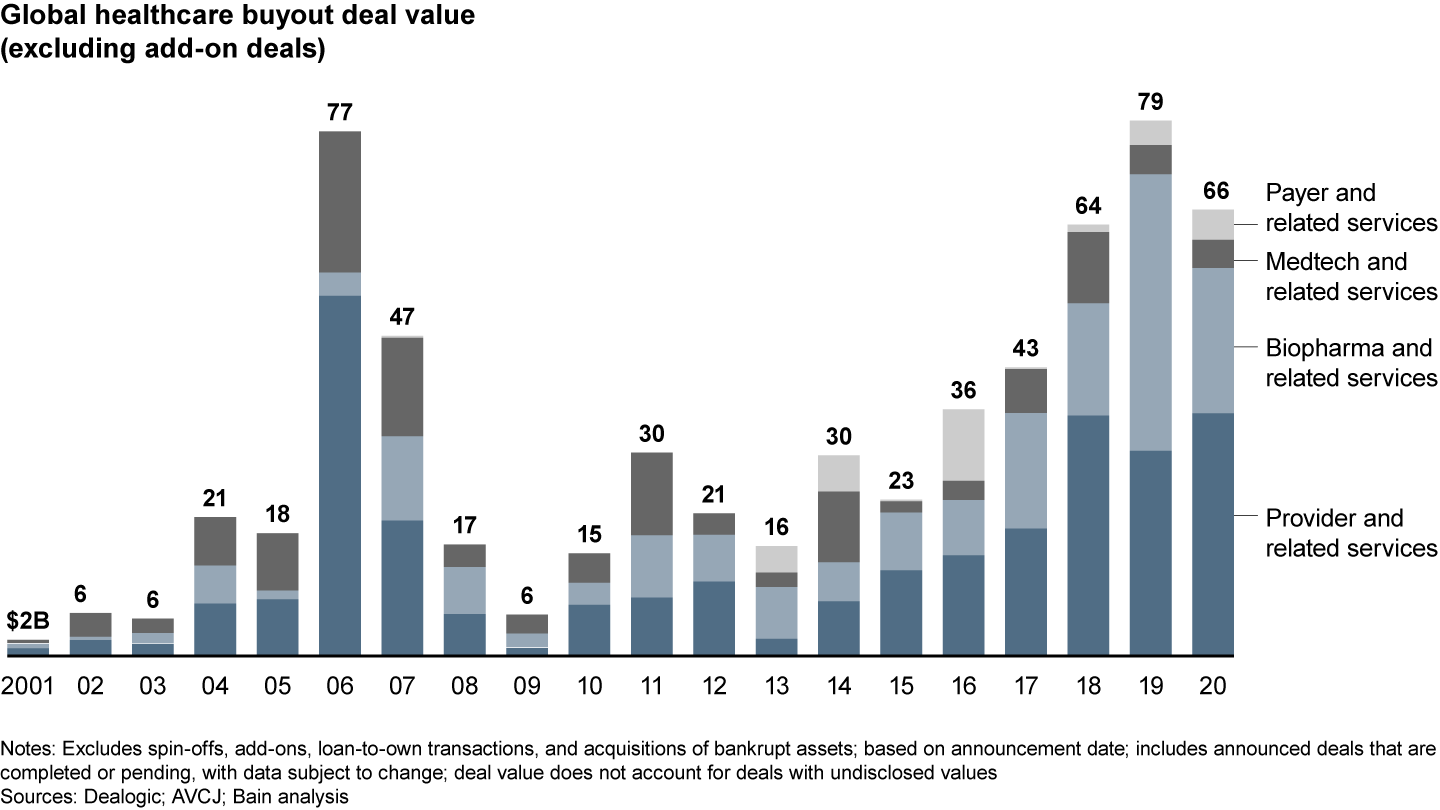
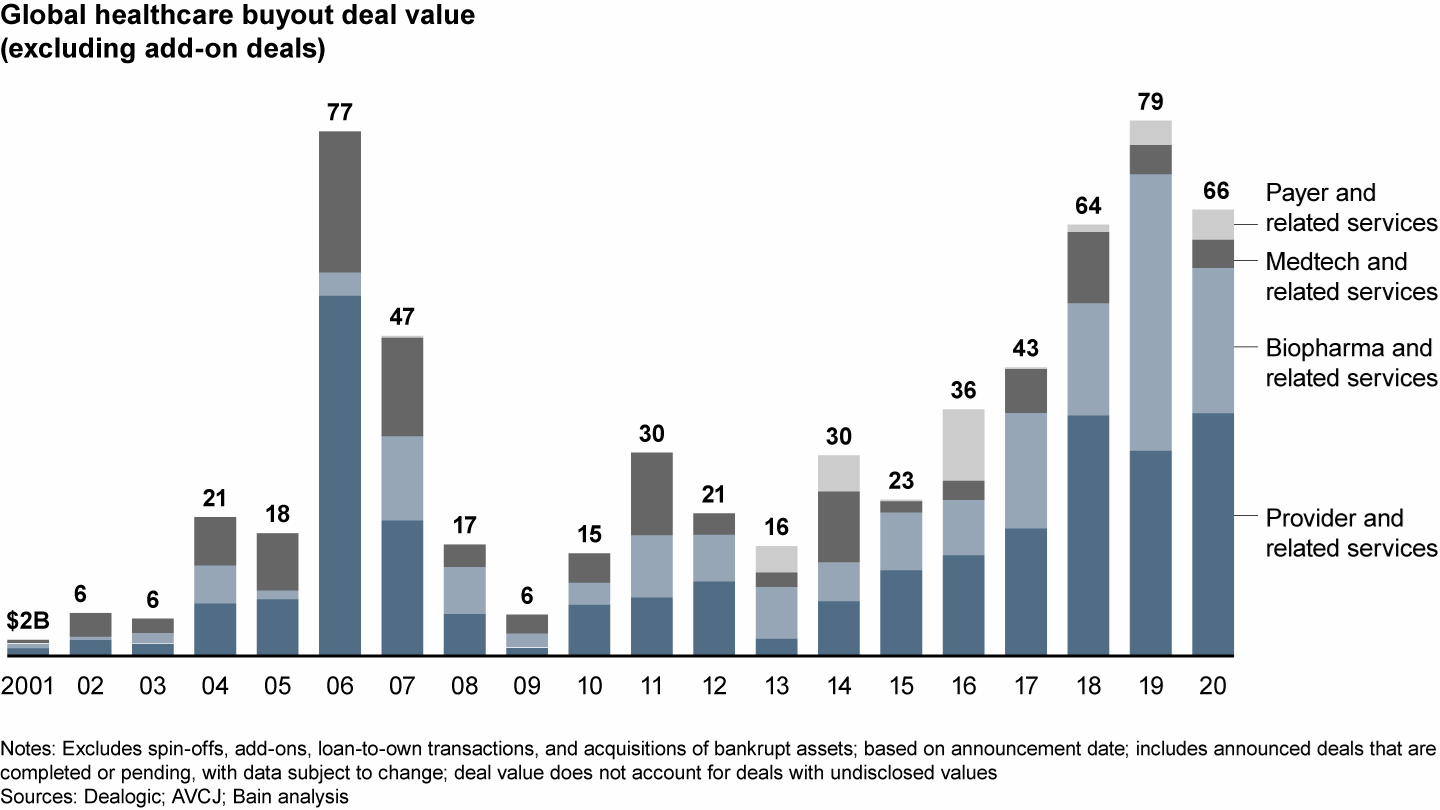
This robust investment performance occurred despite the Covid-19 disruption to clinical trials and commercialization, suggesting that the underlying fundamentals of biopharma remain intact. Funding of investments in therapeutics, as well as M&A activity by corporates, remain strong, with highlights including Gilead’s $21 billion acquisition of Immunomedics and AstraZeneca’s recently announced acquisition of Alexion for $40 billion.
From a geographic perspective, the Asia-Pacific region drove the highest total disclosed value—$9.3 billion or 44% of global value—and volume at 86 deals. Deals in this region tended to be relatively small, and included a number of earlier-stage investments, often in structures that look more like venture deals.
Meet the members of Bain’s Healthcare Private Equity practice.
North America’s activity notched 37% of global value at $7.9 billion. It leaned heavily on biopharma services, including commercialization services, packaging and manufacturing, and specialty contract research organizations (CROs).
Europe, meanwhile, tallied 19% of total value at $4.1 billion with some large deal values remaining undisclosed. The region saw a number of large specialty pharma deals, with more activity likely to come in 2021.
Services flourished in North America and Europe
Although Covid-19 had a negative effect on clinical trials, biopharma deal volume did not suffer as much as other healthcare sectors, and there was appreciable deal flow despite difficult conditions. In particular, supporting services in North America and Europe remained an avenue for investors who wanted to invest in pharma but avoid molecule-specific risk.
Commercialization support services, in particular, thrived during the year, with a variety of transactions focused on companies supporting sponsors across capabilities including medical affairs, patient support services, payer services, marketing agencies, and distribution. We expect this trend to continue given the importance of sponsors realizing strong returns on approved products and given growing numbers of smaller biopharma sponsors with unique commercialization needs. For instance, Harvest Partners invested in ConnectiveRx (formerly PSKW), a provider of patient engagement and market access and adherence support for specialty and branded biopharma manufacturers.
Services investments also gravitated to R&D and manufacturing platforms, supporting biopharma’s strong clinical and preclinical stage pipeline. One example was Blackstone’s $2.3 billion majority stake acquisition of Precision Medicine Group from Berkshire and TPG Growth. Precision is a biopharma service provider focused on oncology and rare disease clinical trial development and drug launch support for smaller sponsors. A majority stake in PCI Pharma Services, a biopharma supply chain solutions provider, was acquired by Kohlberg and Mubadala in partnership with Partners Group. And THL and Frazier backed Adare, a leading CDMO platform formerly backed by TPG. Huntsworth Health (acquired by CD&R for $0.7 billion) and Fishawack (acquired by Bridgepoint) represent just two of the noteworthy pharma services deals completed in Europe in 2020.
Modernizing clinical trials
Clinical trial processes continue to be reinvented and Covid-19 further exposed weaknesses in traditional trial approaches, notably those that depend on in-person patient visits to key opinion leaders for enrollment and monitoring.
The pandemic forced sponsors to deploy virtualization tools and strategies to keep their clinical trials running. This transition shifted recruitment, participation, data capture, and monitoring away from physical sites toward telehealth visits, remote patient diagnostics, and electronic monitoring. Some elements of this line of innovation, such as telehealth trial consults, will no doubt continue after the pandemic abates. Others, such as fully virtual studies, may require further experimentation before a winning model emerges.
In addition, the continued modernization of trials continues to focus on efficiency improvements through deployment of next-generation clinical operations tools, such as e-clinical outcome assessment and e-consent, as well as use of artificial intelligence in protocol design, patient identification, and risk management.
Finally, in some cases, collecting real-world outcomes data to prove efficacy and safety profiles, and to support approval, reimbursement, and usage decisions by stakeholders, mitigates the challenges of trial size, through synthetic control arms; it could also replace the need for new trials. This data plays a growing role in the development process alongside clinical data. For instance, Carlyle Group acquired a majority stake in TriNetX based on the promise of the company’s cloud-based platform for real-world clinical evidence that supports CROs and drug developers.
Europe looks to specialty pharma
Specialty pharma has long been a key target for pharma, with investors seeking commercial-scale, defensible assets. These typically compete in therapeutic areas that have less large pharma investment (such as neurology and antibiotics) and that have some differentiated intellectual property or capability that provides a competitive impediment (small-volume indications or nonstandard dosages). Assets drawing attention this year in Europe were predominantly category leaders, such as Neuraxpharm, acquired by Permira, and Covis Pharma, acquired by Apollo.
Strong adjacency interest for cell and gene therapy
Strong preclinical- and clinical-stage pipelines in cell and gene therapies have investors in Europe looking for ways to participate through suppliers, manufacturing firms, and support services. However, competition for assets with differentiated positions is fierce. Strong corporate interest in the space—as with Catalent’s acquisition of cell therapy manufacturer MaSTherCell—has limited opportunities for private equity sponsors to buy assets with differentiated positions, with Warburg’s coinvestment with ArchiMed in Polyplus being one of the few successful examples.
Asia-Pacific’s growing appetite for direct-molecule-specific risk
For most developed markets in the Asia-Pacific region, biopharma activity resembled that of other regions: a focus on services and stable, mature targets such as Takeda’s consumer healthcare division, acquired by Blackstone for $2.3 billion. However, China and developing Asian markets have shown greater appetite for direct molecule investment.
Following government regulatory actions to encourage local biotech innovation, a number of emerging firms offer attractive prospects, especially to local investors. For instance, Hualan Biological Engineering, a Chinese biopharma developer that raised $290 million from a group of investors led by Hillhouse Capital, focuses on bacterial vaccines for human use.
A bullish outlook for services and high-growth segments
Even in the face of the pandemic, biopharma’s outlook for investment opportunities remains quite bullish. Robust funding for therapeutics suggests a continuation of strong pipeline trends. The major source of uncertainty lies in governmental drug pricing and reimbursement action in the US, though it is unclear whether anything significant for the biopharma industry will materialize.
Services and healthcare IT will remain the most fruitful territory in the US and Europe. We expect especially strong interest in a few segments with high projected growth: cell and gene therapy support, specialty CROs supporting niche therapeutic areas for smaller biopharma sponsors, commercialization services, clinical trial reinvention tools and services, bioanalytical testing services, and real-world data and evidence providers.
Specialty pharma could spark additional interest in certain niches where biopharma has less focus. Infectious disease-focused firms have historically struggled to create a sustainable business model, but there is reason to hope that these areas could become more economically sound after Covid recedes, as the world prepares for future pandemics. Further, we expect to see opportunities with firms that are repurposing technology or capabilities that were advanced during Covid-19, such as mRNA therapeutics, diagnostics, and vaccines, and now could be applied to other indications.
More from the report
-
Welcome Letter: Fertile Conditions for Healthcare Private Equity Investment
-
Healthcare Private Equity Market 2020: The Year in Review
-
The Covid-19 Paradox: Widespread Repercussions for Demand, but New Healthcare Investment Opportunities as Well
-
Healthcare Private Equity in North America: Bring On the Gem Assets
-
Healthcare Private Equity in Europe: Steady Dealmaking despite Many Deferrals
-
Healthcare Private Equity in Asia-Pacific: Riding a Wave of Domestic Innovation
-
Healthcare Providers: New Roll-Up Candidates and a New Look for Risk-Bearing Providers
-
Healthcare Payers: A Bid to Reduce Costs for Patients and Employers
-
Biopharma: Commercialization Support Services Are Thriving
-
Medtech: Four Themes Fueled Deals despite the Pandemic
-
Healthcare IT: Technologies Help Improve Patient Experiences at Lower Costs
-
Healthcare M&A: A Pandemic-Induced Slowdown in Every Sector
-
Healthcare Exit Activity: Robust Capital Markets Spur a Surge of IPOs
-
Healthcare Private Equity Outlook: 2021 and Beyond