Brief
 }
}
Executive Summary
- The global pharma sector over the past decade has demonstrated solid growth of about 5%.
- A group of European mid-sized players outpaced the market, nearly doubling growth by gradually transforming their portfolios, injecting innovation, and accessing the US market.
- Mergers and acquisitions (M&A) have been critical, intentionally targeting rare-disease assets and fueled through the cash generated from disciplined management of legacy business.
- These mid-sized players unlocked rising profitability while increasing spending in research and development (R&D).
- In parallel, outperformers revisited their operating model and upgraded capabilities to play in the new global and innovative field.
In 2025, the global pharmaceutical market reached $1.7 trillion, growing at a robust CAGR of about 5%. Small and mid-sized companies now account for roughly one-third of this market, with about one out of five based in Europe.
Over the last decade, five macro-trends have reshaped global pharma:
- Pharma innovation is accelerating, driven by AI-enabled R&D, new modalities, and precision medicine, opening new therapeutic areas and reshaping value pools.
- Obesity has emerged as the defining near-term growth engine, with GLP-1s anchoring pipelines and catalyzing broader moves into metabolic disease and longevity spaces.
- To keep pace, companies are increasingly relying on M&A and licensing to access innovation, particularly mid-stage assets and differentiated platforms.
- The global innovation landscape has seen the rise of China as an innovation hub, consolidating a US-China duopoly, while Europe risks relative decoupling and a more downstream role as political strategy lacks and investments are derailed.
- Policy reforms are accelerating global price rebalancing, increasing pressure on US prices, narrowing gaps with non-US markets, and reinforcing the shift toward highly differentiated innovation and more disciplined global launch strategies.
As the global market evolves, the European market has seen a distinct growth trajectory among mid-sized players. Some of these players have begun driving incremental growth through innovation, willingly challenging conventions, pursuing new geographies, and building differentiated portfolios that span established and emerging therapeutic areas.
These mid-sized EU pharmaceutical players have deliberately reshaped their business models to use inorganic strategies, embrace innovation, and build meaningful exposure within the US market. They adapted their portfolios, made targeted M&A choices, and pursued growth in areas where their capabilities could make the greatest impact.
As a result, they grew nearly twice as fast as peers that maintained traditional operating models and 1.5 times faster than the sector average, simultaneously doubled EBITDA margins while sustaining higher R&D spending.
The two paths: legacy anchors vs. portfolio shifters
When comparing mid-sized pharma peers by performance and exposure to key variables (innovation, US presence, and R&D), two subgroups emerge (see Figure 1). The first comprises the “legacy anchor” players, which remain grounded in their historical portfolios and geographic footprints. Their commercial models are steadily tuned to European dynamics, and their product mix continues to skew toward established primary and specialty care assets with limited innovation. This strategy has generated stable performance but offers limited upside. Over the past decade, these steady players grew aligned with market evolution and lower profitability margins.
Note: Pharma companies were assigned a score from 1–5 for each KPI based on their percentile positioning; each KPI has been weighted to compute a final growth driver and key performance score denoting legacy anchors vs. portfolio shifters
Sources: Annual reports; Evaluate Pharma; IQVIA; Lit. search; Bain analysisThe second group, “portfolio shifters,” is defined by transformation. These evolving players pursued an intentional shift in both portfolio composition and geographic exposure. They increased their share of innovative assets, primarily through rare disease drugs and other innovative therapies, and they expanded into the US market, where these categories are better rewarded in terms of access and patient penetration.
The portfolio shifters acquired capabilities they historically lacked, including US commercial infrastructure, US-specific market access and medical expertise, business development engines suited for innovation-led growth, and the R&D muscle required to internally develop innovation and manage more complex portfolios. The incremental progress of portfolio shifters vs. legacy anchors is linked to this evolving strategy, as US exposure and portfolio innovation justify the change in pace (see Figure 2).
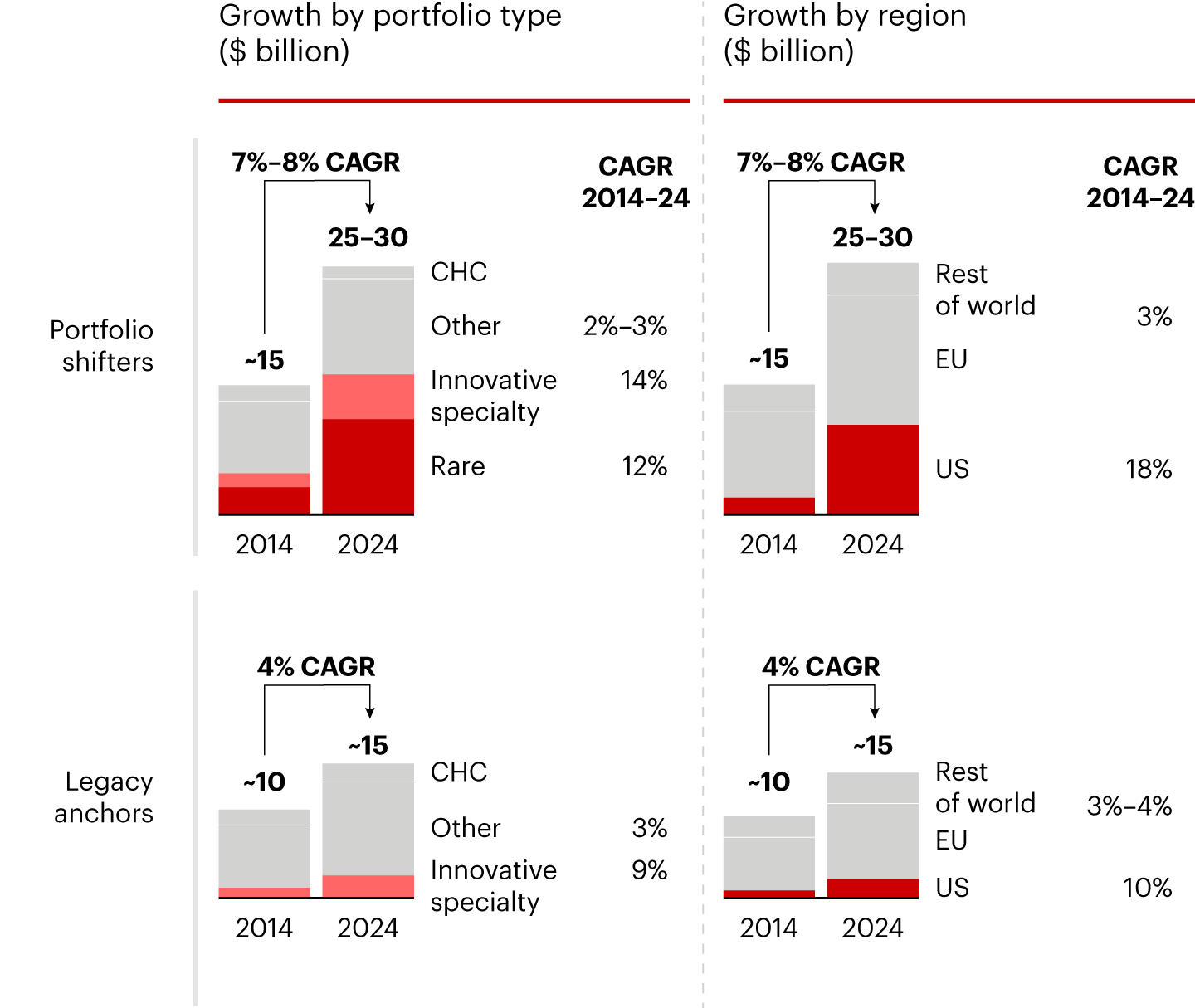

Notes: Rare refers to rare diseases, typically those affecting fewer than 1 in 2,000 people; CHC is consumer healthcare; Other includes non-innovative specialty and primary care
Sources: Annual reports; Evaluate Pharma; IQVIA; Lit. search; Bain analysisThe performance gap between the two groups is clear: Those that increased exposure to the US and innovated their portfolios have outgrown the global pharma market, growing at about 8% CAGR, 1.5 times faster than the sector average and nearly twice as fast as European legacy anchor counterparts, which grew below sector average at about 4% (see Figure 3).
Profitability tells a similar story. Portfolio shifters lifted EBITDA margins by about 12 percentage points to reach almost 30% in 2025, compared with the 19% margins and 5–7 percentage point improvement seen among legacy anchor players.
As portfolio shifters moved toward innovation, they gradually increased R&D spending (18%–20% of revenues versus 10%–12% for legacy anchor counterparts), as part of a coherent set of strategic choices. This deliberate shift toward an innovation-driven strategy was built in a stepwise approach to preserve financial sustainability rather than achieve one-off wins.
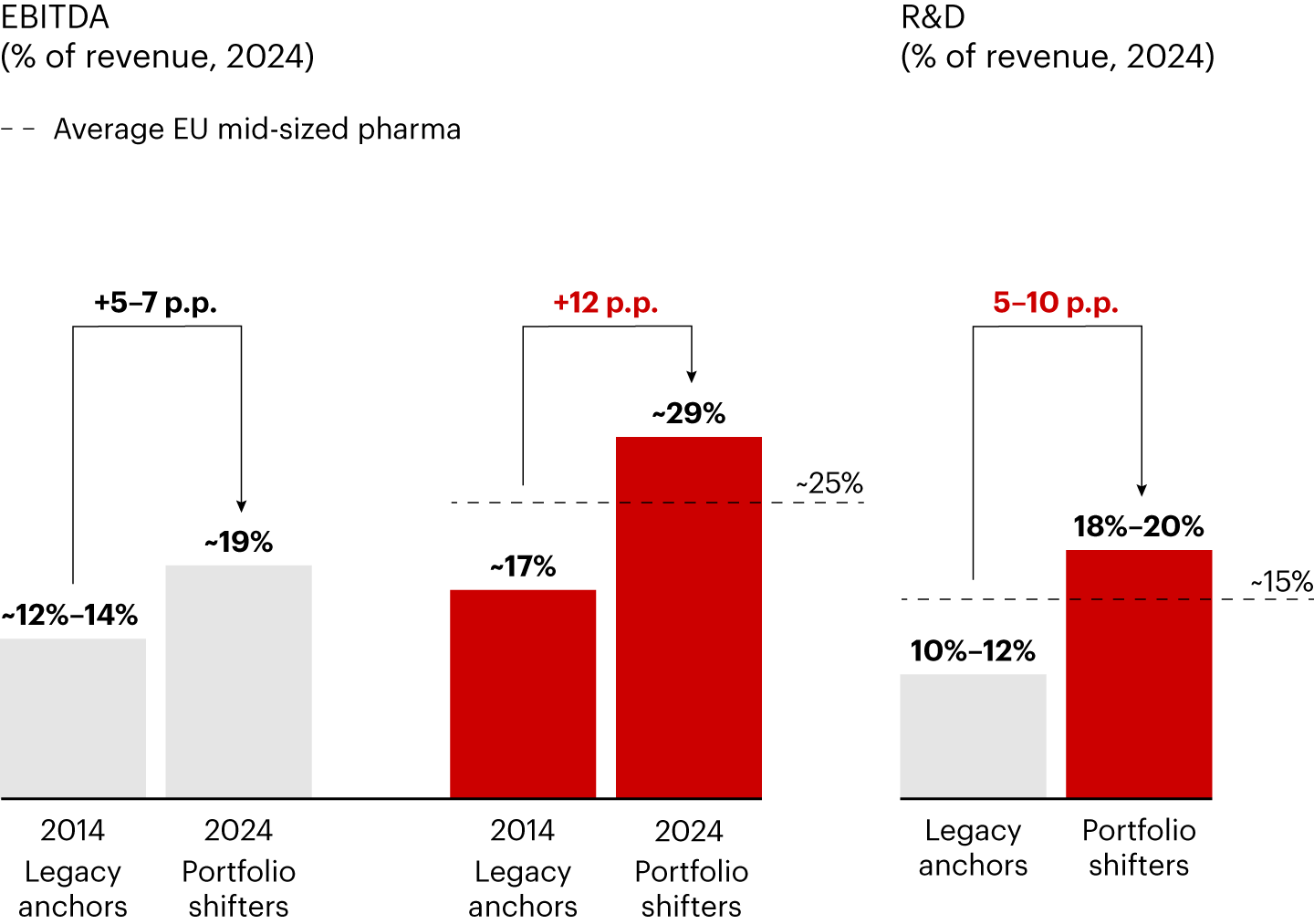

This evolution provides a compelling blueprint for how mid-sized European pharmaceutical companies can overcome structural stagnation and improve long-term momentum. There are notable challenges, but the players already moving in this direction are showing progress is possible and financially sustainable, as it brings improved profitability margins.
Key growth challenges and best practices for evolution
Within Europe, innovation on its own seldom achieves optimal economic outcomes. Factors such as structural pricing dynamics, fragmented reimbursement systems, and limited scale restrict the potential of advanced innovative assets across the region. For this reason, mid-sized European pharmaceutical companies have coupled portfolio innovation with strategic geographic expansion, acquiring platforms in the US, where greater scale, more robust incentives, and favorable market conditions offer stronger rewards for innovation.
For most EU-based mid-sized pharmaceutical companies, the push toward innovation and US expansion is not without constraints. They begin with fewer financial resources than large global competitors and often lean on heritage portfolios reliant on established drugs, leaving limited room to absorb risk, fund major shifts, or withstand multi-year development timelines. Also, many mid-sized players lack the in-house R&D scale and capabilities to generate innovative assets on their own and have little or no commercial presence in the US.
Within these limitations, portfolio shifters have deliberately embraced best practices that allowed them to innovate their portfolios and enter the US in a financially sustainable way. M&A is the key enabler to unlocking the US-centric innovation-driven play, as it allows mid-sized European players to leap-frog peers (see Figure 4). Acquisitions provide the most rapid and risk-contained path to innovation and geographic expansion, but not all assets are fit for purpose.
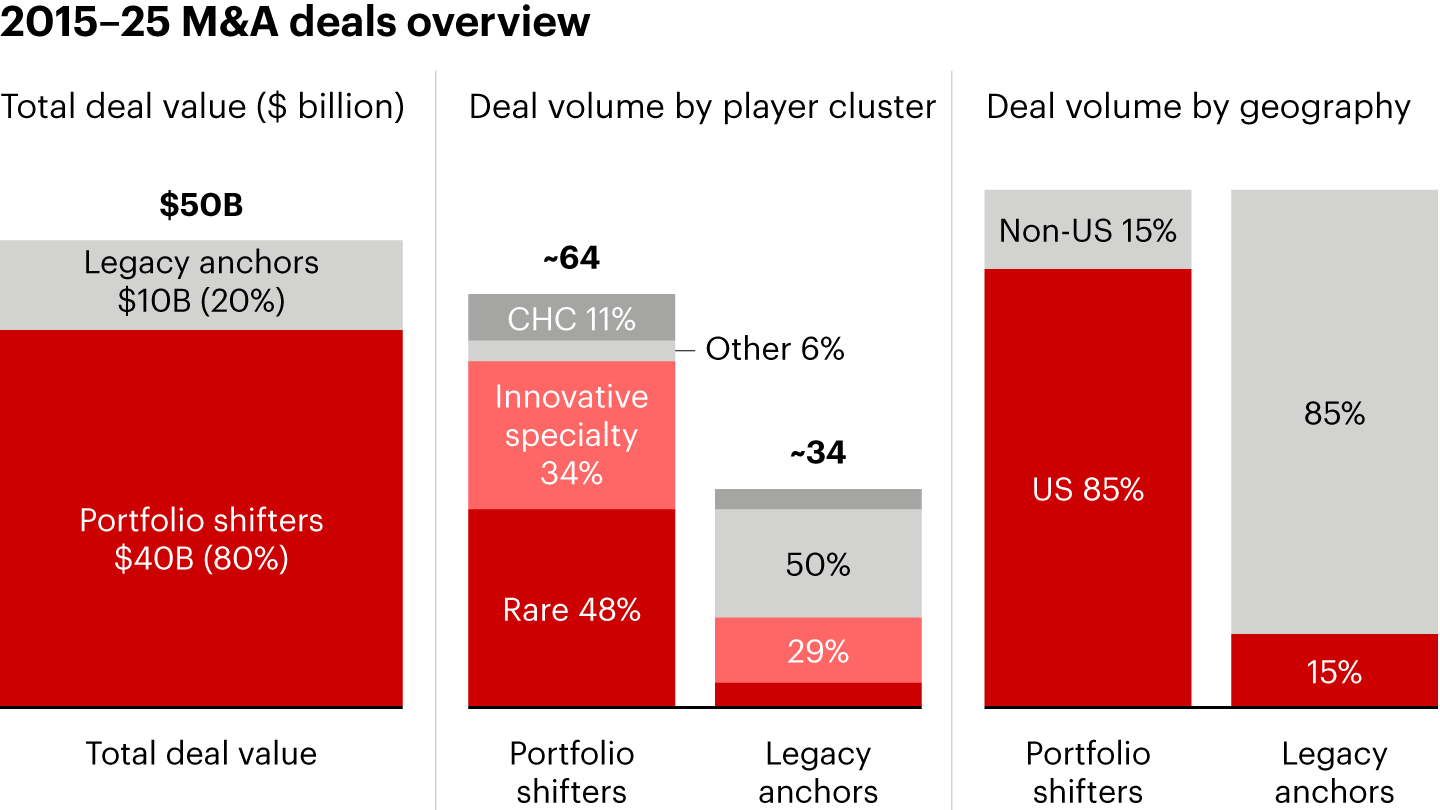

Notes: Geography perimeter also includes deals with key focus in the US that also have a commercial footprint in the EU; Rare refers to rare diseases, typically those affecting fewer than 1 in 2,000 people; CHC is consumer healthcare
Sources: Dealogic; Annual reports; Bain analysisThree insights help define a successful US entry strategy:
- Specialty care is structurally too big to play for mid-sized players.
- Rare disease offers the clearest “right to win” in the US.
- It’s best to start by acquiring a marketed asset to anchor the platform.
Avoid broad specialty care plays: Although attractive in theory, US specialty markets are structurally challenging for mid-sized players. Competing effectively requires sizable sales organizations, continuous R&D investment, and heavy commercial infrastructure. These requirements are difficult to sustain without substantial scale or a narrowly defined area of specialization.
Use rare disease as the entry point: Rare disease represents a fit-for-purpose foundation with focused prescriber bases, limited competition, and supportive orphan-drug incentives and regulatory pathways. This combination allows mid-sized European pharmas to deliberately establish a presence in the US in line with their portfolio strategy and company identity, without confronting the full operational and competitive intensity of specialty care.
Start with a marketed asset: Best practice shows that an initial acquisition should deliver immediate revenue and EBITDA, minimizing development and launch risk while establishing commercial capabilities. A commercialized asset provides both a financial anchor and an operating foundation that can later be used to pursue earlier-stage innovation.
In practice, this strategy often results in a dual-portfolio model: heritage products that sustain scale and cash flow in Europe paired with a focused, innovation-led rare-disease presence in the US to drive growth. While a fully organic path is possible, it requires significantly more time and capital and carries materially higher R&D and execution risk, making M&A-led entry the more pragmatic route for most mid-sized EU players.
What EU mid-sized pharma leaders should do now
As pressure on healthcare budgets continues to rise in Europe, the performance gap between portfolio shifter and legacy anchor players will continue to widen.
While the effects of recent policy reforms are still unfolding, the US is expected to remain a cornerstone of global pharmaceutical innovation. European policymakers will face increasingly difficult trade-offs: Either preserve access to cutting-edge therapies by moving pricing closer to US levels—simultaneously optimizing resources on established products—or accept a potential slowdown in the introduction of new treatments across Europe. Under either outcome, these pressures are likely to weigh on the core business models of mid-sized pharmaceutical companies and further underscore portfolio innovation and US’s presence as critical engines for lasting EU mid-sized growth.
In this context, European leaders are being asked to think about their companies’ strategic evolutions:
- Question the current strategic model and evaluate how to participate in the global innovation market if this is not yet a company priority.
- Plan for a staged evolution, as this journey will not happen overnight. It requires patience, discipline, and a very intentional, well-articulated inorganic strategy. Each step must be carefully sequenced so it creates the conditions for the next, with a clear understanding of limits to preserve financial stability and enable lasting success.
- Adopt a disciplined dual-portfolio strategy, maximizing value from heritage assets through rigorous portfolio and capital allocation discipline to fund and focus investment in innovation-led growth engines, while preserving financial sustainability.
- Redesign your operating model and capabilities to equip the company with the appropriate tools and resources to play on a different field. Ensure US-centric commercial and market access capabilities, reinforced R&D and medical functions, and a business development function capable of driving your intentional inorganic strategy agenda.
While the core ingredients of this model are increasingly evident, Bain’s direct experience supporting these transformations shows there is no single winning formula—success depends on tailoring the strategy to each company’s starting point, ambition, and capabilities.
As competition for quality assets accelerates and the window for differentiated US entry narrows, the winners will be those that move early and execute flawlessly across the entire transformation journey: from clearly defining inorganic strategy, intentionally selecting areas of interest and phasing priorities, to appropriate asset scouting and valuation, all the way to effective management of post-merger integration.
The insights presented in this brief are based on Bain's extensive experience supporting mid-sized pharmaceutical companies in developing growth strategies and assessing market dynamics over several years.