Brief
Sales of pharmaceuticals in China are already the third-highest in the world, and China is likely to become the world’s second-largest drug market within the next year. Multinational pharmaceutical corporations cannot afford to underperform in China if they want to achieve global prominence. But as significant policy and market changes rock the basis of competition, incremental adjustments to the current commercial model will be insufficient to maintain a growth trajectory.
The Chinese government’s commitment to improved healthcare access is creating millions of new pharmaceutical customers. However, the government’s need to contain costs has increased pricing pressure on drugs, which now threatens the profits that multinational producers of “originator” drugs1 have long enjoyed. Merely tweaking the existing commercial model will not be enough to manage the inevitable price decline. Without changing the current business model, Bain & Company analysis shows a doomsday scenario in which the current existing originator model goes “un-economical” within five to seven years (see Figure 1).
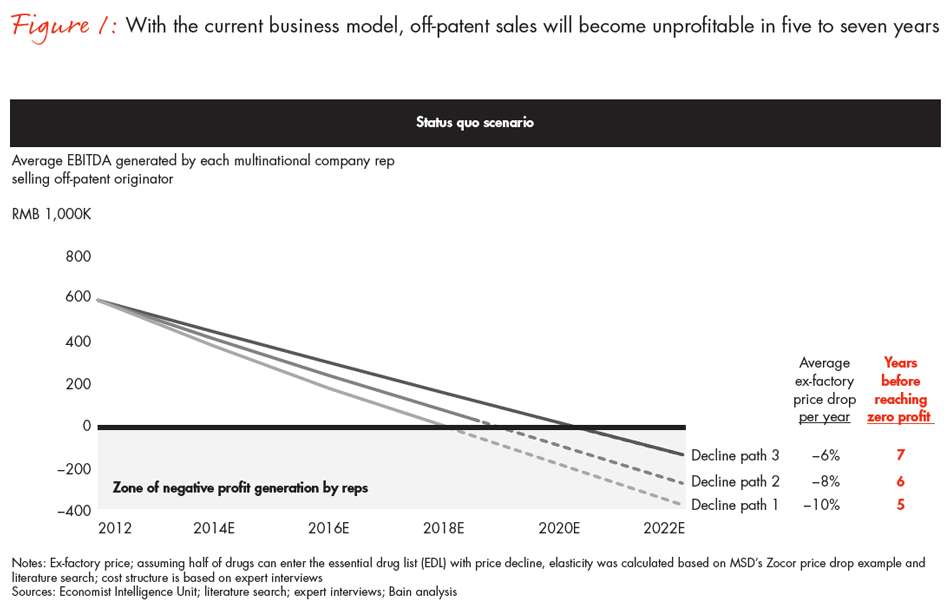
Success will require a fundamental reinvention of the commercial model. For multinational corporations (MNCs) to stay competitive with increasingly savvy local producers, they will need to introduce innovative products tailored to Chinese health needs, accompanied by continued expansion of established products to meet more basic demands. The best way to support this fit-for-local business will be to restructure the sales function, apply a more sophisticated approach to market access that involves stronger relationships with payers and patients, and promote a smarter use of digital technology. A strategically coherent and integrated commercial model can ease the pain of price reductions and protect the bottom line.
China’s current healthcare system has created an addiction to drugs by Chinese hospitals and physicians. Until now, pharmaceutical sales have constituted 40% or more of the revenue—and almost all of the profit—of Chinese hospitals. The government aims to alter the incentive system with a zero percent drug markup policy in hospitals across the country by 2015. This commitment has stripped the autonomous power of prescription away from hospitals and physicians, threatening to steadily erode their income.
In April 2014, Zhejiang province, one of China’s richest, adopted zero percent markup for all hospitals within its jurisdiction, and others are following suit. The government plans to offset that loss of drug-related revenue by increasing the fiscal subsidy to hospitals and adjusting medical service charges, but it’s not yet clear how that will unfold.
All that is happening against the backdrop of an ethical compliance crisis that has the capacity to accelerate the changes. No longer can hospitals thrive primarily on drug revenues. No longer can physicians use drug sales to reinforce their income. And no longer can pharmaceutical companies engage physicians in the same way without incurring more stringent scrutiny.
As if all these systemic changes were not sufficient to raise business concerns, the disease burden in China, especially in non-communicable diseases such as diabetes, cancer and heart disease, is growing significantly. Patients too often have to pay a major portion of medical costs, and as they learn more about the effectiveness of non-reimbursable drugs, they are likely to demand better access to them.
Building a new growth platform
If MNCs can no longer afford to continue with a commercial model largely built on originators and salesforce reach and frequency, how will they stay and thrive in China? As these forces challenge the existing originator business, MNCs urgently need to build a new growth platform or have an exit strategy ready for implementation.
Originators still represent a significant part of MNCs’ overall revenue base. The biggest growth opportunity for originators in China will now be in the broader market being created by the expansion of social insurance, where unmet needs are huge. China has 23,000 hos-pitals and more than 70,000 urban or rural community healthcare centers. Most MNCs today cover only a fraction of this delivery network.
The role of sales representatives
A new business model will likely still be based on the salesforce, but companies must find a way to boost their productivity in a broader market. Sales representatives in China are not cheap, but they have been considered worth the investment. An experienced rep can bring in 2.5 to three times top-line sales returns, with a net profit of one times the bottom-line return. Frequent rep visits (a Bain survey indicates 70% of physicians have a weekly contact with a sales representative) have reaped rich profits for MNCs. However, with increasing cost per unit of sales and declining prices, some MNCs are starting to experiment with commercial partnerships and outsourcing to better afford the required additional coverage.
Build out medical affairs
Government scrutiny of the sales-physician relationship has caused leading MNCs to shift more resources to the medical affairs function, prioritizing quality over quantity of dialogue with physicians. Medical affairs will play a more proactive role with physicians than in the past, while working closely with market access teams to address the increasingly sophisticated needs of government payers for outcome data.
These shifts will not be without organizational challenges. Medical affairs staff will have to emphasize customer service as well as academic knowledge, and companies will need to learn how to better attract and retain the additional medical expertise this expanding market-place requires.
Enhancement of market access capability
Market access teams have been relatively comfortable with three main functions: creating and maintaining personal relationships with key officials, interpreting existing government regulations, and managing pricing and reimbursement. But market access professionals will likely grow in importance as the government payer becomes more sophisticated and private insurance emerges as another source of funding. These teams will need to use their technical expertise to enhance their roles beyond the personal level and become true institutional partners with regulators. They will also need to consider how to more effectively engage with patients on treatment and value choices.
Improved digital technology
New market demands for a broader but cost-effective geographic impact will require a more effective use of a variety of digital tools. A recent Bain report showed China eclipsing the US in 2013 as the world’s largest e-commerce market, demonstrating the transformative power of the Internet.
Even though Chinese physicians are spending more time online for professional purposes, digital tools have yet to be utilized effectively by MNCs to improve salesforce management, educate patients or substantively enhance physician-detailing efforts. There is little doubt that the use of digital media to support information dissemination and patient adherence efforts has tremendous potential in China, where patients are already using the Internet for disease and treatment self-education.
A fit-for-local innovative portfolio
Multinational pharma companies must also make a decisive pivot toward innovative products to satisfy China’s real gap of unmet medical needs and to continue enjoying the attractive economics associated with exclusivity. Like the West, China suffers from a dramatic increase in diabetes, hypertension and the resulting complications. In addition, a significant proportion of Chinese are at risk for problems in the respiratory system, liver and stomach, given the air pollution and prevalence of viral hepatitis and Helicobacter pylori infections.
From a development perspective, this trend offers MNCs the opportunity to give China higher priority via more parallel trial designs for speedier launch, earlier engagement in the launch and patent process, tailored trial protocols to fit the Chinese standard of care, and development of commercial partnerships in China to spread the cost and risk of innovation.
While managing the glide path for originator products, MNCs must balance two management strategies: a more aggressive shift to new, patented products and a better way to sustain the continued growth of off-patent originators as price erodes over time (see Figure 2). MNCs might consider building strategic alliances to either pool resources and go after a broader market, or acquire or invest in a local company with complementary products and capabilities.
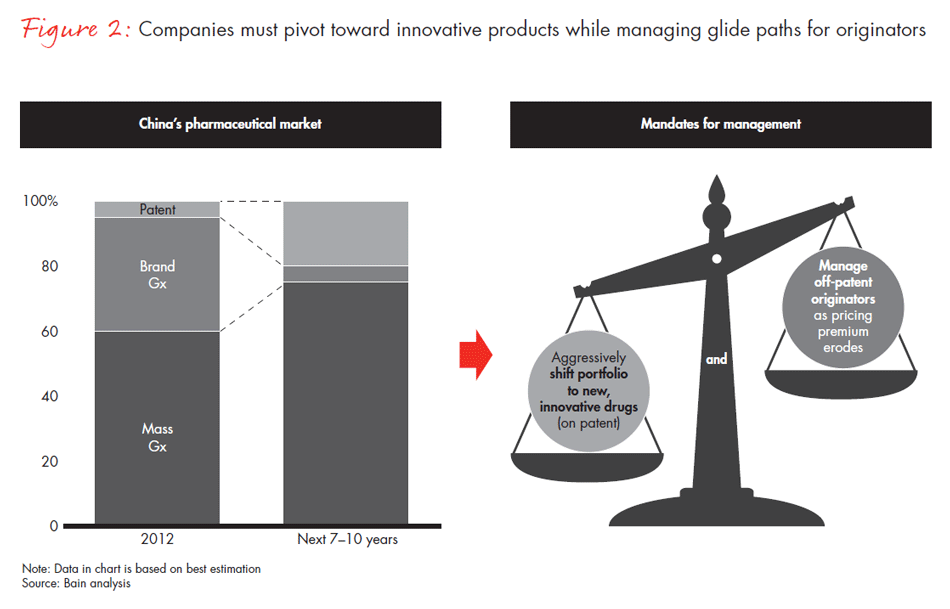
Many leading MNCs have renewed their commitment to China in light of these market changes. In March 2014, Eli Lilly announced a new $323 million investment to open its third insulin plant in China. This followed Bayer’s nearly $140 million investment to boost production capacity in Beijing and its proposed acquisition of China’s Dihon Pharmaceutical, announced in February 2014.
Finally, the private sector now offers serious commercial consideration. The government is encouraging the growth of private insurers and providers, including those that are foreign-owned. The goal is to have the private sector account for 20% of available hospital beds by 2015. Private providers and insurers have the advantage of no legacy-distorted incentive issues and no public tender purchasing of drugs. They are more rational economic buyers of pharmaceutical products, which may result in a diminished role for sales reps but an increased need for better access data and stronger key account teams and customer service capabilities.
Even with these new opportunities, if companies decide these options do not provide a path they want to pursue, or if the economics of change become untenable, then an exit strategy may become necessary. There are relatively few options for such an alternative. But companies can consider retreating to a more defensible core where products are more accepted and less costly to maintain, or transferring off-patent assets to a strategic local alliance and turning to a commission and licensing business model.
Embracing new challenges is never easy, but opportunities in the Chinese market are too significant to leave to the status quo. As you prepare your strategy, it may be useful to answer some key questions:
- Given the changing regulatory and competitive landscape, how will you defend your existing business while ensuring 100% compliance?
- The stakeholder landscape is shifting. Who are the priority regulatory, payer, provider and patient groups to whom you will need to pivot in the coming years? How will you differentiate your capabilities?
- As the landscape changes, there will be an increased need for local, regional and global pharmaceutical leadership to collaborate more effectively. What actions will you take to help all management layers navigate these changes successfully?
- China is still a market with tremendous growth potential. Will you be able to compete on innovation, control costs and stay in the originator business longer than your competitors?
- What will success look like for you in five years?
1 Originator drugs are branded pharmaceutical products made by the company owning the initial patent for the underlying compound, after the patent’s expiration.
Philip Leung is a partner with Bain & Company’s Shanghai office and leads Bain’s Greater China Healthcare practice. Grace Shieh is a partner based in Bain’s Los Angeles and Shanghai offices. Ellon Xu is a principal based in Bain’s Shanghai office.

