Brief
 }
}
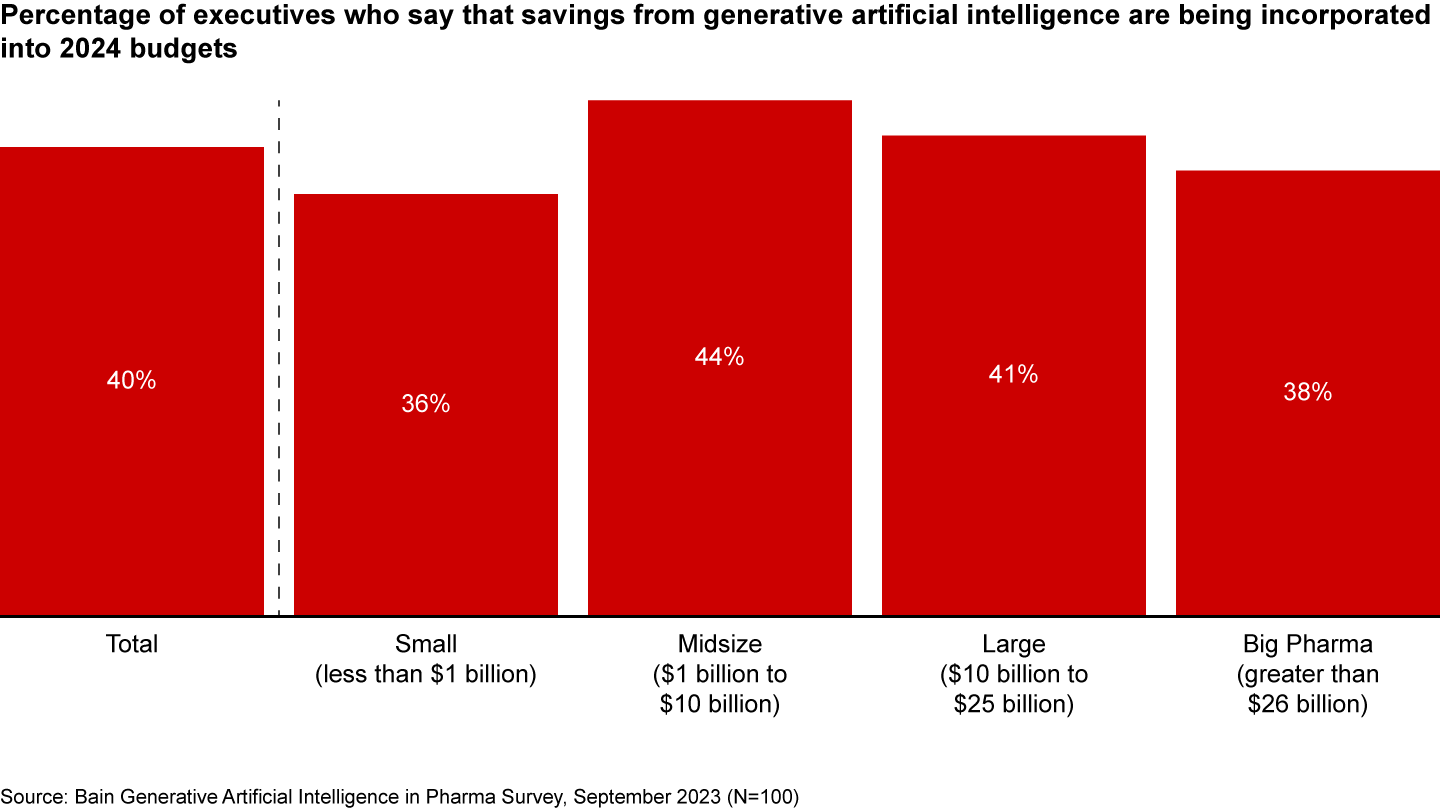
The generative artificial intelligence (AI) transformation is well underway in pharma. And pharma companies have high confidence in its value: Already, 40% of executives say that they are baking expected savings into their 2024 budget (see Figure 1), and 60% have set targets for cost savings or productivity boosts, according to a recent Bain survey.
Nearly 60% of executives say that they have moved beyond ideation and brainstorming to building out use cases. In fact, 55% reported that they expected to have multiple proof-of-concept or minimum viable product builds by the end of 2023.
With companies large and small making significant headway in realizing the benefits of generative AI, what will separate the best from the rest? Over the next three to six months, the companies that make the greatest progress will be the ones that move from isolated pilots to scaling winning use cases across the board. These leaders will pull away from the pack with an operating model that supports fast growth at scale and prioritizes the most valuable opportunities.
The state of AI in pharma
Classical data science and machine learning are nothing new to pharma executives who have been investing in productivity enhancements for years, primarily in the drug discovery space. Bain research shows that 54% of pharma companies have automated biomedical literature review solutions, and 46% are using AI as part of their process to find potential disease targets.
Now, generative AI is broadening the aperture of use cases with new opportunities across the value chain. Biomedical literature review and preclinical research remain among the most popular use case areas, although we’re also seeing high investment in IT and competitive intelligence (see Figure 2). Within these top areas, more than 60% of executives, on average, say that they have at least a proof of concept in development, and around 10% have already rolled out tools.
These early adopters have moved swiftly, often reaching a working pilot within about eight weeks. And already, many are seeing tangible value.
For example, within six weeks, one healthcare leader was able to develop a working pilot of an AI-enabled chatbot to help answer pharma reps’ medical questions on a subset of its products. It significantly boosted contact center agent productivity by improving the number of issue resolutions per hour. Similarly, Eli Lilly estimates that it has saved around 1.4 million hours of rote human activity since 2022 through automation and technology. With further AI investments, it aims to reach 2.4 million hours by the end of the year.
Other leading pharma companies have made rapid gains in a range of areas, from research and development to support functions. One created an accurate model for clinical trial patient identification in a quarter of the time needed for previous machine learning models. Several have succeeded with generative AI tools that draft summaries of regulatory filing content or responses to regulator questions. Others have focused on chatbots for knowledge management, enabling employees to quickly query internal documents.
Some have pursued commercial endeavors: For instance, companies are already piloting generative AI to streamline salesforce tasks, including dynamic content generation, and one company is using AI to draft custom ad copy according to US Food and Drug Administration guidelines.
The level, speed, and success of subscale experimentation has been impressive. But as early wins breed interest and energy across the organization, it’s increasingly critical for executives to shift from disconnected pockets of generative AI experimentation to an enterprise-wide program. Otherwise, the organization can trip over itself, becoming the bottleneck to its own potential.
Industry pioneers haven’t just built two to three proofs of concept. They have scaled those proofs of concept and encouraged adoption. They have also created thoughtfully structured backlogs with use cases throughout the company prioritized by how much value they bring and how practical they are to develop.
The best enterprise-wide roadmaps group use cases in thematic clusters, outlining the intent to evolve them over time. Leaders are starting with low-risk use cases and launching them in safe environments, with the ambition to test, learn, and gain confidence before going live with more mature, disruptive solutions. For instance, a company may prioritize an internal knowledge management chatbot before evolving it into an external-facing chatbot using similarly unstructured data. Or a company may begin with a patient-facing solution that relies on a human to mitigate risk, with the aim of eventually creating a fully automated version.
For example, Syneos Health, which has a multiyear deal with Microsoft to leverage OpenAI’s GPT technology, brought together a team of data scientists and business function leaders to create centralized, reusable machine-learning building blocks. In addition to working on a chatbot that can search across 400,000 clinician protocols, the biopharma company says that it is exploring applications across the value chain, from clinical trials to marketing platforms. Sanofi is also laying the groundwork for AI at scale by launching Plai, an app that harnesses internal data across the organization to generate predictions and simulations, giving decision makers a comprehensive view of the company’s activities and insights.
To support a prioritized roadmap, industry leaders are also ensuring that they have the appropriate technical backbone in place. Many have signed contracts with multiple generative AI foundation model providers to experiment and understand the nuanced differences in their performance.
How to scale generative AI
If pharma companies want to generate value from generative AI as fast as the technology allows, they need to ensure that the organization is ready. Companies can take a three-tiered approach to prime their operating model for generative AI at scale:
- Determine your strategic posture. Leading organizations will establish decision-making and funding models that prioritize high-return use cases. In particular, they will ensure that those use cases fit within their investment themes around bold bets for the future of the business. When it comes to funding, organizations often bill generative AI investments to IT, although they typically deliver the anticipated savings to the respective functional budgets. Winning companies won’t let these conflicts stand in the way of adoption. Instead, they will find ways to incentivize business unit leaders to invest in disruptive, value-generating AI initiatives.
- Lead through change. Generative AI at scale requires strong internal leadership and cross-functional alignment. The best companies will establish an organizational center of gravity with several executives who act as generative AI champions. The team holds the organization accountable to its roadmap and decision-making model.
- Build the foundations. In addition to the right technology, data, and models, generative AI at scale requires reorienting the organization to support big visions.
- Reshape your talent strategy. Many pharma companies continue to struggle with hiring data scientists for AI initiatives. Given the shortage of talent with generative AI expertise, companies that want to be included among the next generation of AI leaders will need to recruit aggressively now.
- Forge strategic partnerships. As they build up their stable of in-house talent, leaders will partner with external vendors for support. Consider, for instance, how Sanofi is using BioMap’s AI platform that converts proprietary data sets into biological maps of proteins to optimize its drug discovery process at scale, or how Bayer is working with Google to automate drafting and translating clinical trial communications in multiple languages.
- Engage on ethics and regulation. Data security, privacy, legal issues, and ethical considerations, such as biases in models’ input and output, require a thoughtful approach from the start. While adhering to guidelines and regulations is paramount, industry leaders will go a step further with a companywide risk management approach, including guardrails that they continuously adjust to ensure safe deployment. For example, GSK has established an in-house responsible AI team that brings together experts in engineering, philosophy, and policy to explore ethical and societal considerations and implement a framework for safe and ethical development. In addition to strict infrastructure and processes, AI users receive training to ensure proper practices.
Generative AI is already top of mind for most pharma companies, with 75% citing it as a C-suite and board priority. And investors are watching closely to differentiate the pioneers from the followers.
As leadership teams move beyond experimentation into pilots and launches, they are thinking carefully about when and how to communicate their AI journey to investors. Those that can signal a structured, scalable enterprise-wide program, rather than a smattering of standalone initiatives, will reap the rewards in the next phase of AI.
