Report

At a Glance
- Record levels of capital flowed in 2021 to the biopharma sector, which returned to a prepandemic growth curve.
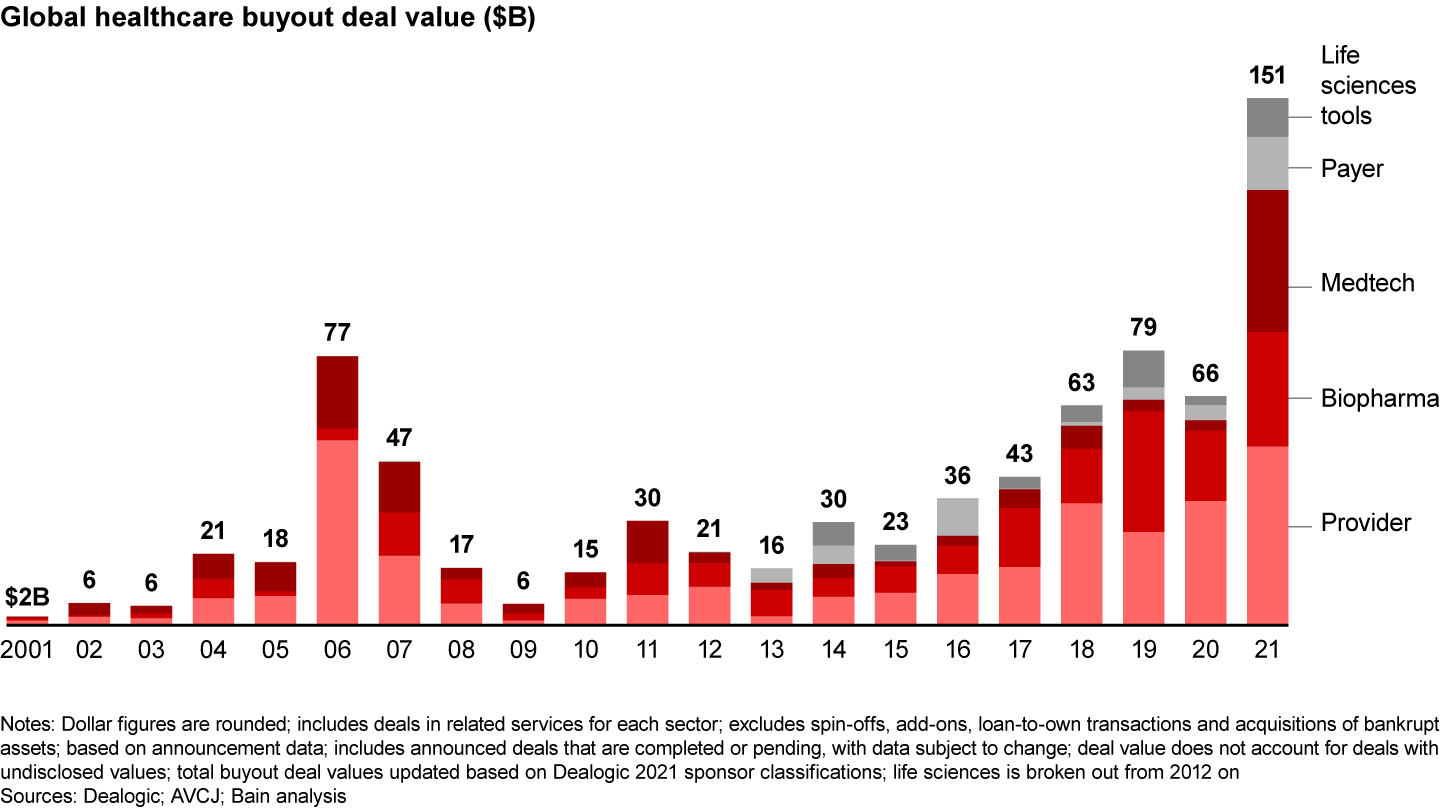
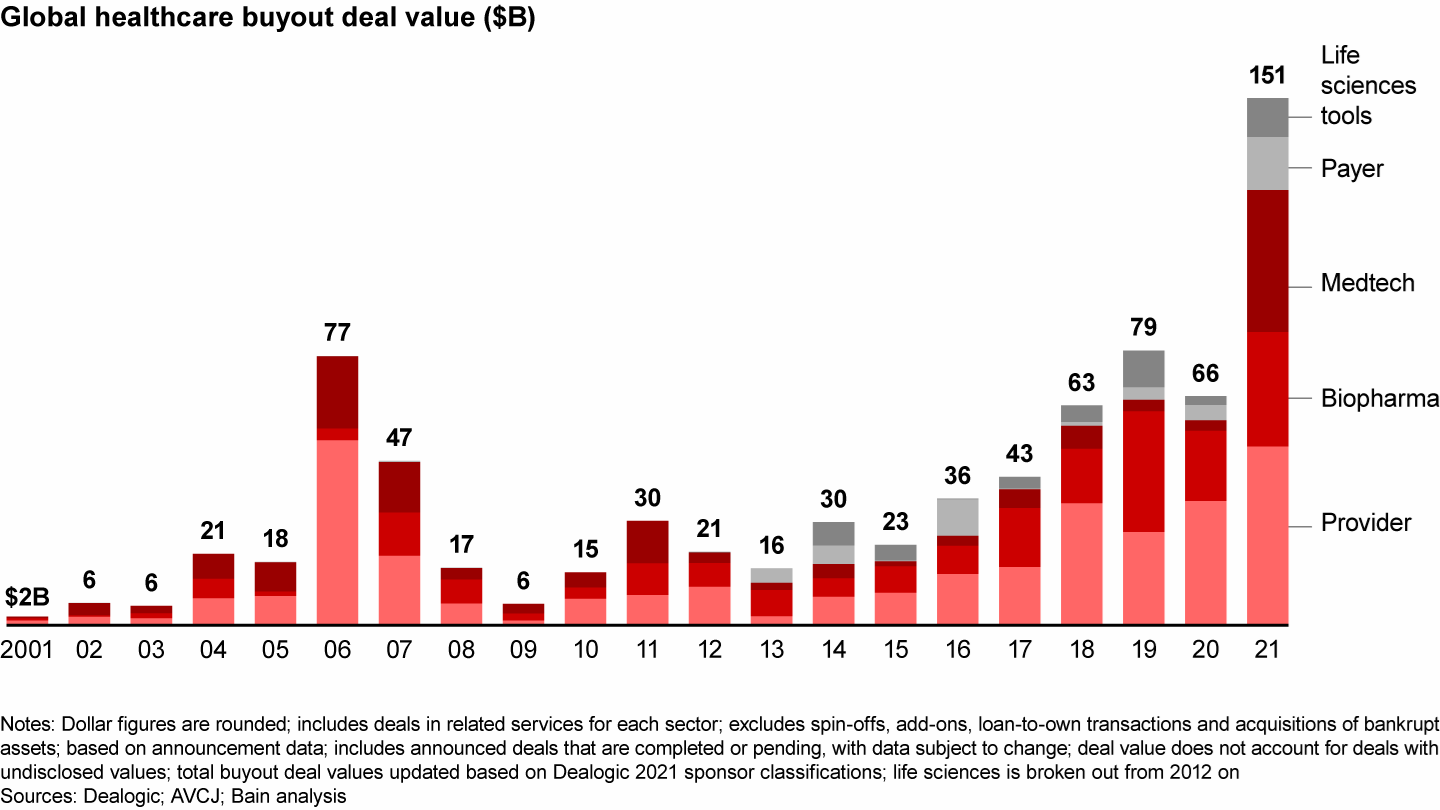
- The number of deals grew modestly, but buyout deal value surged about 65%.
- Investors continued to gravitate to traditional pharma services assets, limiting clinical and pricing risk.
- Strong fundamentals, along with the rise of Covid-19-specific therapeutics, should create ample opportunities in the years ahead.
This article is part of Bain's 2022 Global Healthcare Private Equity and M&A Report
In contrast to the market uncertainties of 2020, strong fundamentals in biopharmaceuticals produced a number of large deals in 2021, especially in Europe. Traditional pharma services led the way. Global deal volume rose slightly to 147 from 137 the year prior, and disclosed value surged to $33 billion from $20 billion (see Figure 1). (Because this report breaks out the life sciences sector for the first time, the 2020 biopharma data varies from last year’s report.)
European deal value more than quintupled to $11.4 billion, on the back of buyouts such as UDG Healthcare at $3.8 billion and Cooper Consumer Health at $2.6 billion. The story was different in the Asia-Pacific region, which continued a steadier growth and maturation of healthcare private equity activity. There, deal volume dipped only 3% from the prior year’s record high. While Asia-Pacific still accounts for 53% of total transactions, private equity sponsors encountered more competition from corporate buyers and capital markets.
One twist during the year was the cluster of attempted public-to-private transactions. However, Advent International and Singapore's sovereign wealth fund withdrew their bid to acquire Swedish Orphan Biovitrum after an insufficient percentage of outstanding shares approved the bid, Reuters reported, highlighting the execution difficulties sponsors can face.
Traditional pharma services remain solid
Covid-19 caused investors to gravitate in 2020 to pharma services assets, particularly in North America and Europe, thereby limiting clinical and pricing risk. This trend continued in 2021, with contract research organizations (CROs), contract development and manufacturing organizations (CDMOs), and clinical site organizations (CSOs) generating significant investor interest.
In a departure from 2020, several large organizations with broad therapeutic or product portfolios were acquired by private equity sponsors. For example, Goldman Sachs and EQT paid $8.5 billion to buy Parexel, a global CRO focused on developing and delivering innovative therapeutics. And Partners Group acquired the CDMO Pharmathen from BC Partners for $1.9 billion. Several CSOs traded, headlined by GHO Capital’s buyout of Velocity Clinical Research, which conducted trials during Operation Warp Speed in the US.
Private equity funds and corporate buyers also targeted niche CROs that serve small to midsize pharma companies, such as Novo Holdings’ purchase of Altasciences, and the WindRose recapitalization of Veristat.
Corporate M&A activity blossomed as buyers sought to enhance drug development capabilities and become more efficient. Large deals include Icon’s $12 billion purchase of PRA Health Sciences to expand and modernize its clinical trial offerings, and Thermo Fisher‘s $17.4 billion (excluding net debt) acquisition of PPD to expand offerings into the lab and CRO space.
IT specialists supporting commercialization and clinical trials
Outside of traditional pharma services assets, specialized services companies that leverage technology to support a specific stage of drug development have seen an uptick in acquisitions, particularly firms that streamline commercialization and modernize clinical trials. These include firms offering software, consulting, data, and analytics.
Market access for pharmaceuticals figured in General Atlantic’s buyout of CareMetx, a specialty patient hub, and the merger of Evaluate (an HgCapital portfolio company) with Managed Markets Insight & Technology (a Welsh, Carson, Anderson & Stowe portfolio company) to strengthen their position in pharma commercial intelligence. The combined entity then acquired Panalgo, a healthcare analytics platform. Commercialization and pipeline consulting services also drew deals. GHO bought Clearview Health Partners; Trinity Life Sciences recapitalized with Kohlberg & Co.; and Clayton, Dubilier & Rice purchased UDG Healthcare, an advisory, communications, commercial, clinical, and packaging services firm, for more than $3.7 billion.
Assets that help clinical trials to operate more efficiently and, in some cases, in a decentralized model, became more relevant during the pandemic. Take software and services that digitalize core trial operations such as patient recruitment. Deals here include Thoma Bravo’s purchase of Greenphire, a provider of financial process automation for clinical trials; Astorg-backed ERT’s merger with Bioclinica, which sells software for clinical trial management and e-consent; and Carlyle’s $430 million investment in Saama, a clinical analytics company that played a role in the fast-tracked Covid-19 vaccine trials.
Capital also flowed to companies that use data analytics to improve the design and execution of trials. Notably, Nordic Capital and Astorg acquired Cytel, a provider of statistical software for clinical trial design and execution. On the growth equity side, Goldman Sachs invested in 4G Clinical, a provider of randomization and trial supply management, and Coatue Management led an investment in Reify Health, a technology platform optimizing patient recruitment, with a valuation of $2.2 billion.
Producers of over-the-counter products and generics
Companies that make over-the-counter (OTC) products or generic medications benefit from low R&D risk, predictable revenues, and the potential for follow-on acquisitions. For example, CVC acquired Cooper Consumer Health, which owns OTC brands, from Charterhouse Capital for $2.6 billion. Perrigo sold its Latin American OTC business to Advent International for an undisclosed sum and its generic business to Altaris Capital Partners for $1.6 billion. And Nordic Capital bought Advanz Pharma, a manufacturer of generic specialty medicines, for $846 million.
Cell and gene therapy innovation
Promising cell and gene therapies pipelines have lured investors looking to participate through derivative plays in suppliers, research tools, and support services. Derivative plays shield investors from direct pipeline risk while exposing them to much of the upside in this rapidly growing field. Clayton, Dubilier & Rice; Merck; and McKesson Ventures made a noteworthy majority investment in M2Gen, which focuses on data and analytics that enable personalized cell and gene therapies to treat cancer.
Some investors, confident in their scientific prowess, opted to take on direct pipeline risk through growth-equity investments. For example, Vitruvian Partners acquired a stake in Oxford Biomedica, a cell and gene therapy company.
A bullish outlook
Structural characteristics of the biopharma industry have only been strengthened by the efforts to address Covid-19. Other factors also should work in the industry’s favor in the years ahead. Aging populations in high-income countries will increase demand for therapeutics. Abundant government, university, and private funding for R&D in many countries has built strong innovation pipelines across therapeutic modalities and disease states. Moreover, the medium-term risk of major drug price reform in the US market has diminished as bipartisan negotiations at the end of 2021 produced a less ambitious reform plan.
Private equity has historically found opportunities in biopharma derivative plays among companies that service biopharma giants, and these opportunities should continue. We expect to see more attention on IT firms that leverage technology to modernize the drug development arc. Technology to modernize clinical trials will also present good prospects as the digitalization of clinical trials comes of age.
Cutting-edge therapeutic modalities, especially cell and gene therapies as well as mRNA, will grow and create openings for deals. As investors gain confidence in their scientific judgment, directly investing in assets with pipeline risk may present unique opportunities for high returns.
Biotech activity held stable in 2021, lagging the broader biopharma sector. Looking ahead, investors will need to be discerning, since public markets signal that valuations may come down from 2020 levels. However, investors should keep an eye out for the next BioNTech or Moderna, as Covid-19 becomes endemic and sustains a huge addressable market for therapeutics.