Etude
Product launches serve as defining moments for pharmaceutical companies to create sustained value. Well-managed launches provide the single most powerful way to offset the revenue shortfalls of the approaching patent cliff. The challenge to meet earnings targets puts even more pressure on product launches for at least two reasons:
- First, the market access environment has become more restrictive. Our assessment of launches made in Europe shows that 60% to 90% face access restrictions by payers and governments due to a lack of perceived differentiation or cost-effectiveness. While the US numbers are somewhat better, insurers and providers will likely impose more restrictions as budgets tighten and the Affordable Care Act is implemented. A recent survey of 100 health plans and pharmacy benefit managers suggested that 37% are likely to support alternative payment models within the next three years.
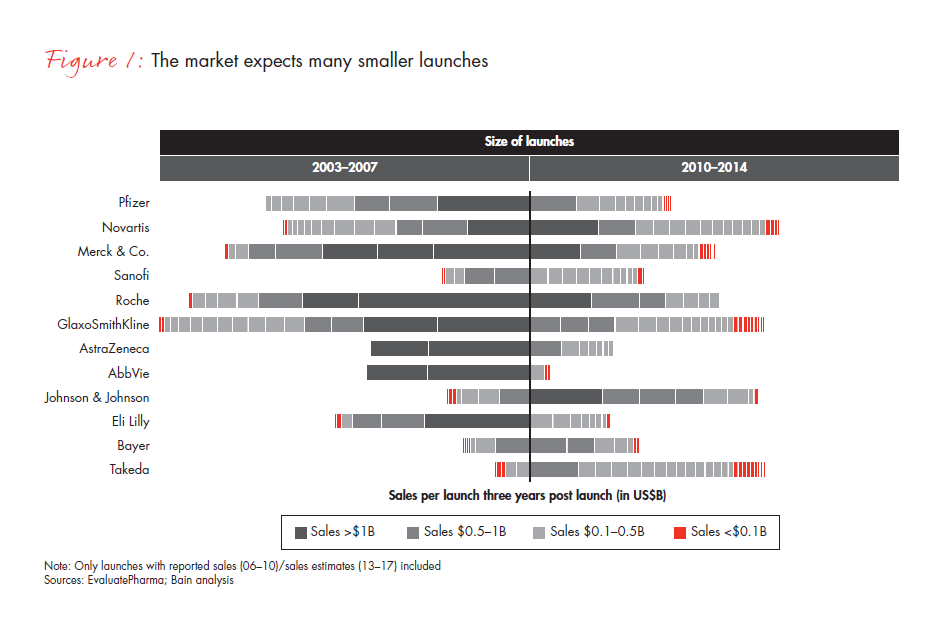
- Second, a different product portfolio mix requires a new approach. While companies used to launch a few large products each year, in-depth pipeline assessments show they now launch many smaller products with more intense competition (see Figure 1).
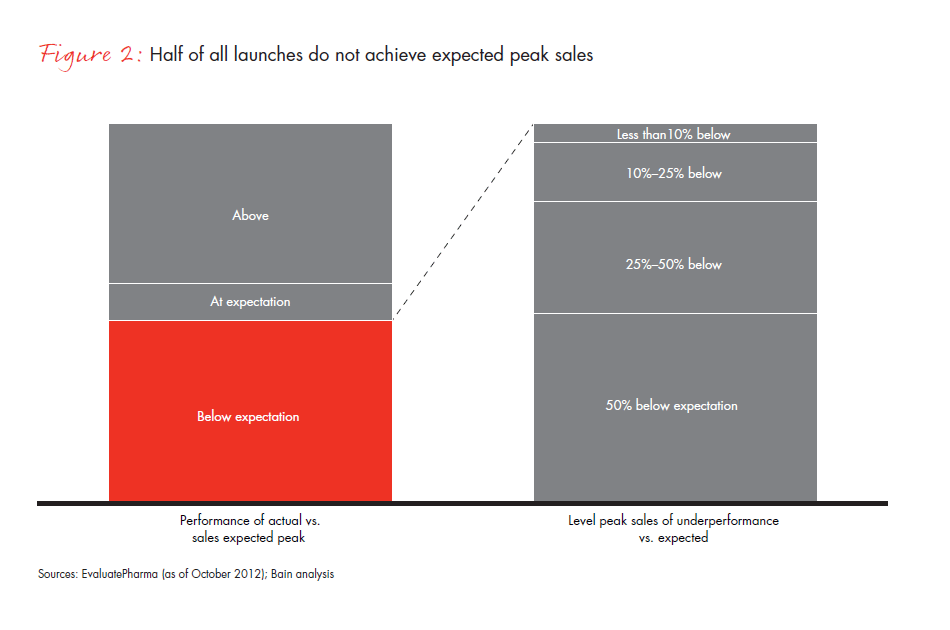
The industry has not yet adjusted its launch approach to the new reality. One indication of this is that half of all launches now in the peak sales range did not meet expected levels, and half of those missed expectations by 50% or more (see Figure 2). In such a challenging environment, pharmaceutical companies recognize that not every launch can be a blockbuster and are searching for new ways to prepare for launch. In our experience, the companies adjusting most effectively to this reality share three common characteristics:
- They are moving away from a one-size-fits-all approach to launch and tailoring activities to four assets or archetypes.
- They are updating their launch toolkits to adapt to a world of smaller launches with more access restrictions.
- They are retooling their organizations into Launch Factories that launch many products continuously and consistently.
Move away from a one-size-fits-all approach to launch
Many companies have pursued a one-size-fits-all approach to their launches when it comes to positioning, go-to-market strategy and resourcing. That approach may still work for blockbuster assets, but it is less applicable to other pipeline drugs.
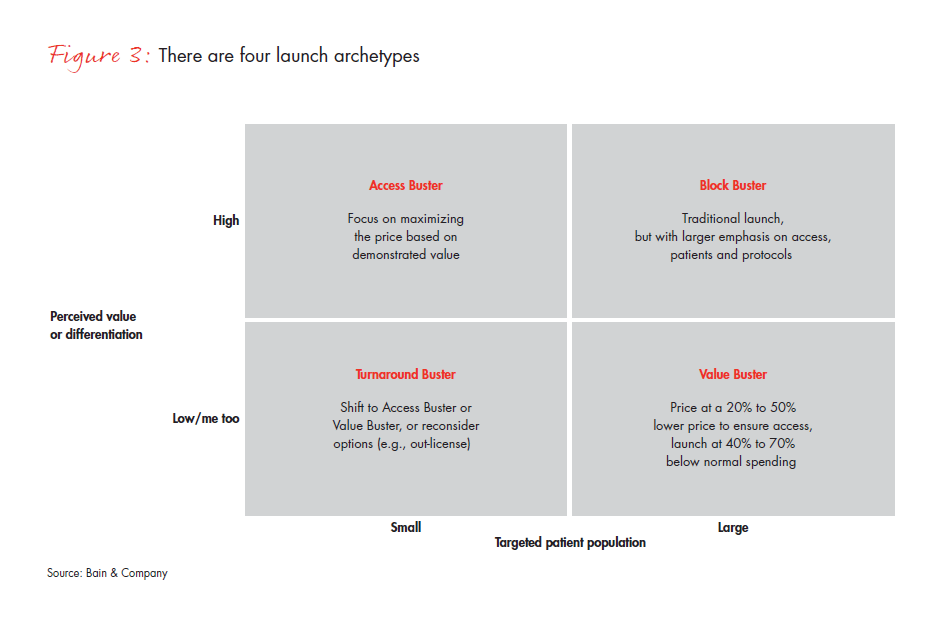
Our analysis looked at many variables that might help differentiate among launches and predict success. The two most meaningful variables were the size of the target population and how payers and providers perceived product differentiation. That insight revealed four distinct product archetypes, each with its own optimal launch approach: Block Buster, Value Buster, Access Buster and Turnaround Buster (see Figure 3). Identifying products on these two dimensions gives companies a practical approach for planning each launch according to the archetype that best describes its characteristics. For example:
- A traditional Block Buster approach will still work for products targeting a large patient population and high perceived value and differentiation, but it will continue to require a focus on efficient launch activities (e.g., Merck’s diabetes drug Januvia, which emphasizes digital channels and patient education along with traditional feet-on-the-street and directto- consumer models).
- For products with a large patient population but perceived low differentiation by payers, pursuing a Value Buster approach could pay dividends, with a price point 20% to 30% lower to ensure access, and resourcing 30% to 50% below the traditional Block Buster (e.g., Sanofi’s colorectal product Zaltrap, which was forced to discount price 50% due to perceived similarity in outcomes to those of a competitor).
- For products with a smaller patient population but a high perceived differentiation, an Access Buster approach will make the most of price based on demonstrated value (e.g., Genentech’s highly successful Herceptin, which, while expensive, has retained its price because it demonstrated successful outcomes for HER2-positive types of breast cancer, with a companion diagnostic).
- The most challenging archetype, which we label a Turnaround Buster, includes products with a smaller patient population and low perceived differentiation. The best strategy for these products may be to refocus for other indications, delay the launch to capture additional trial outcomes or simply consider other options, such as out-licensing (e.g., Rare Disease Therapeutics’ Anascorp, a drug for scorpion bites with wide price variation that caused payer backlash).
Our assessment of the late-stage pipelines of nine large pharmaceutical companies, covering 89 products and 140 indications, shows that most companies have products distributed differentially across the four archetypes. This varied product distribution reinforces the critical importance of a tailored approach that takes into account the characteristics of each launch type (see Figure 4).
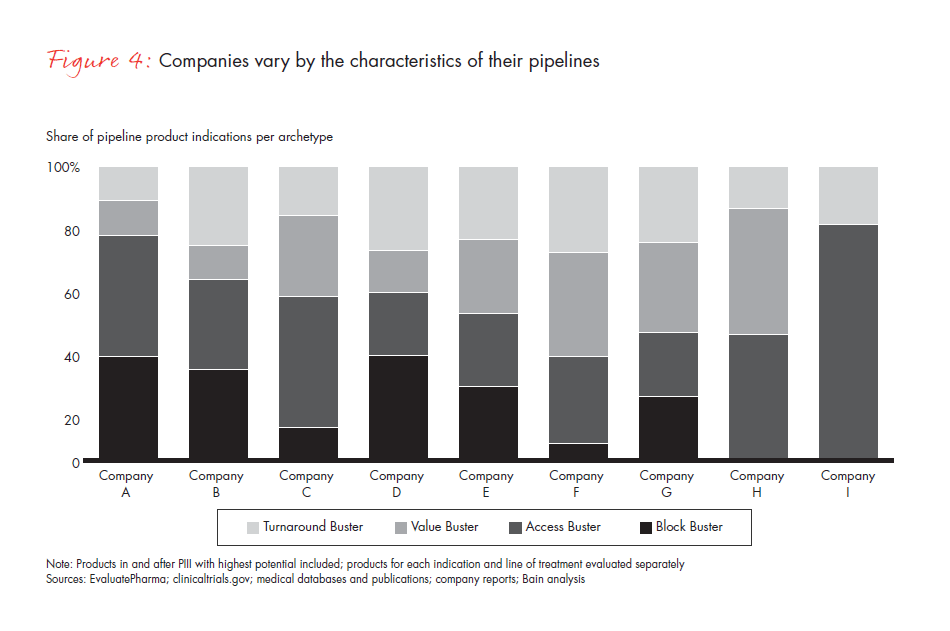
The power of the archetype approach emerges more fully as companies balance their commercial focus across the four launch types and shape strategies accordingly. The key launches in each pipeline may be broadly spread across all four approaches or narrowly focused on one or two. For example, one company found that while its pipeline had two classic Block Buster candidates, its other pipeline assets were spread over the remaining archetypes. As a result, the company developed a differentiated approach and institutionalized a global launch SWAT team targeted to their most important markets and regions. In addition, it ramped up access capabilities across the most relevant markets to account for the distribution of launch assets across archetypes.
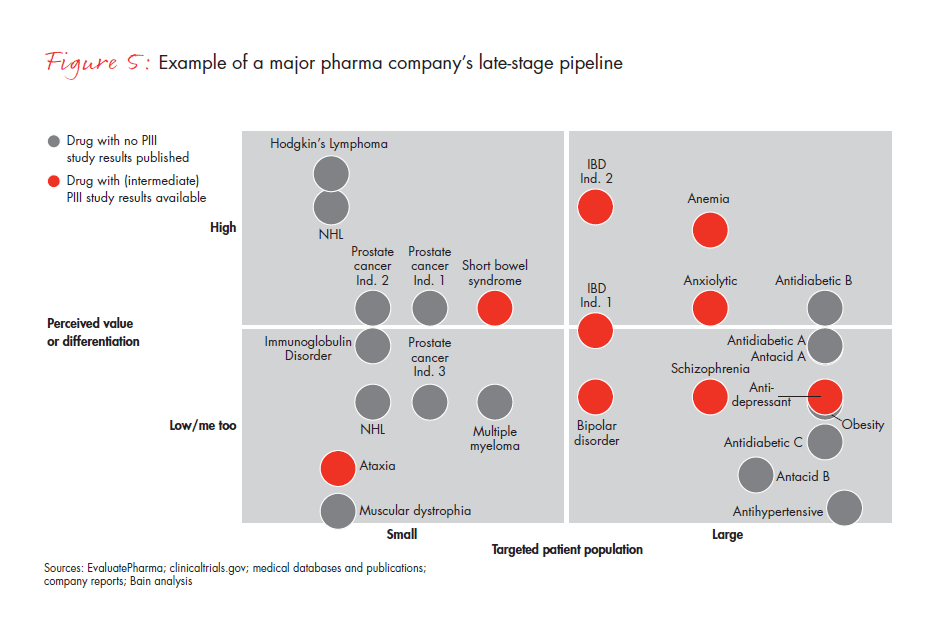
Another major company discovered that its late-stage pipeline products fit all four archetypes, triggering a different set of questions (see Figure 5). Its Block Buster products may generate confidence in the launch’s success, but the team will need to be sure it knows how to make the most of the opportunity. The company’s Value Buster product, with a potentially large target population but issues of value differentiation, will pose other challenges, such as whether there is a potential lower-cost approach that will still keep the product competitive. The Access Busters raise questions of how to ensure maximum access for a limited target group. These products can demonstrate value through post-launch trial commitments, real-life data, outcome-based pricing and geographic selection, but it also will help to emphasize clinical positioning to Key Opinion Leaders (KOLs) and Phase 2 preparedness. Its Turnaround Busters, with a small target population and low perceived differentiation, will be the most difficult to address and will require a fall-back option.
Update the launch toolkit
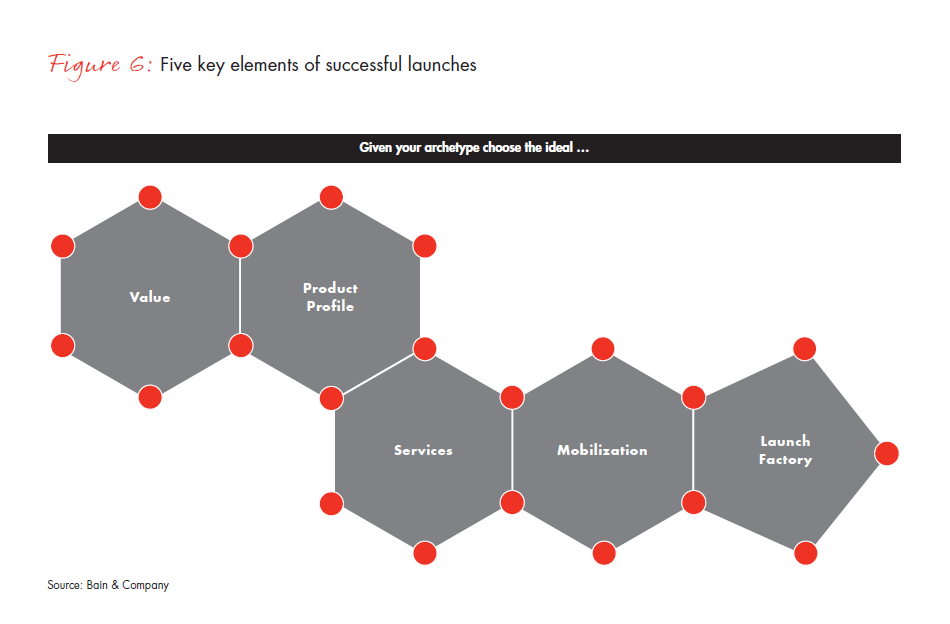
After identifying the appropriate archetype for each launch, companies can move to launch preparation. At this point, there are large opportunities to add significant value beyond the conventional approach of shaping product positioning and checklists. The basic toolkit for a successful launch has five elements: Value, Product Profile, Services, Mobilization and Launch Factory (see Figure 6). While all of these elements need to be in place for every launch, their emphasis and actions differ significantly across archetypes (see Figure 7).
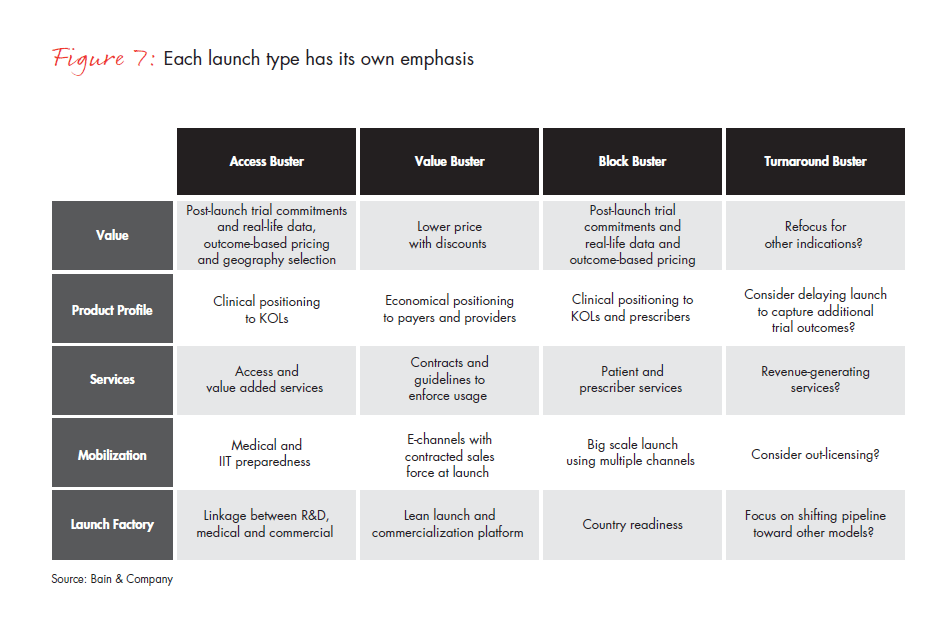
Companies must demonstrate the product’s Value to the payer to obtain the right price and reimbursement. The basic requirements include a reimbursable dossier, standard prices and successful management of payers, regulators and health technology assessment organizations. A new product launch capability, however, may require additional tools, such as first indication choice/ delay, post-launch Phase IV commitments, real-life data to support differentiation, alternative pricing approaches and a broader focus on advisers to regulatory agencies.
The best Product Profile tends to emphasize a comparison of the clinical profile vs. in-market products and forecasting based on internal research and development projections. Additional levers require assessing the clinical profile vs. competitors’ pipeline products and using more objective forecasting by independent analysts.
Typical approaches to outreach and Services are KOL programs and patient advocacy engagement. Emphasizing different patient services (e.g., funding support, new compliance approaches), provider services (e.g., local budget/capacity models) and more sophisticated care management and data platforms for integrated care services will strengthen this approach.
Nitin Chaturvedi, a partner in Bain's Healthcare practice, breaks down the macro trends that are making traditional go-to-market approaches in the healthcare industry less effective and outlines tactics needed to achieve successful pharmaceutical drug launches.
For Mobilization, the typical approach will focus on salesforce preparedness, the reallocation of resources from other therapeutic areas or the inclusion of launch costs in each country’s budget. More productive options might include focusing on building critical skills/ resources for each therapeutic area, hiring 50% of staff from competitors for new therapeutic areas and dedicating budgets and incentives from 24 months before until 24 months after launch.
Finally, the Launch Factory fundamentally restructures the current product-by-product approach. Building a Launch Factory adjusts the company’s organization to allow it to continuously and consistently launch many smaller products, as we describe in further detail below.
Retool your organization into a Launch Factory
Launching 5 to 10 new products each year poses a different challenge from launching a Block Buster every other year. The steps outlined above give companies a practical framework to address the new requirements for each individual launch. With many diverse products and regions to consider, however, executive teams also need a repeatable process that can guarantee consistent outcomes. The opportunity here is to build a Launch Factory. This approach requires a commitment to change the structure and roles, governance and decision making, financial incentives and capability management across the entire organization (see Figure 8).
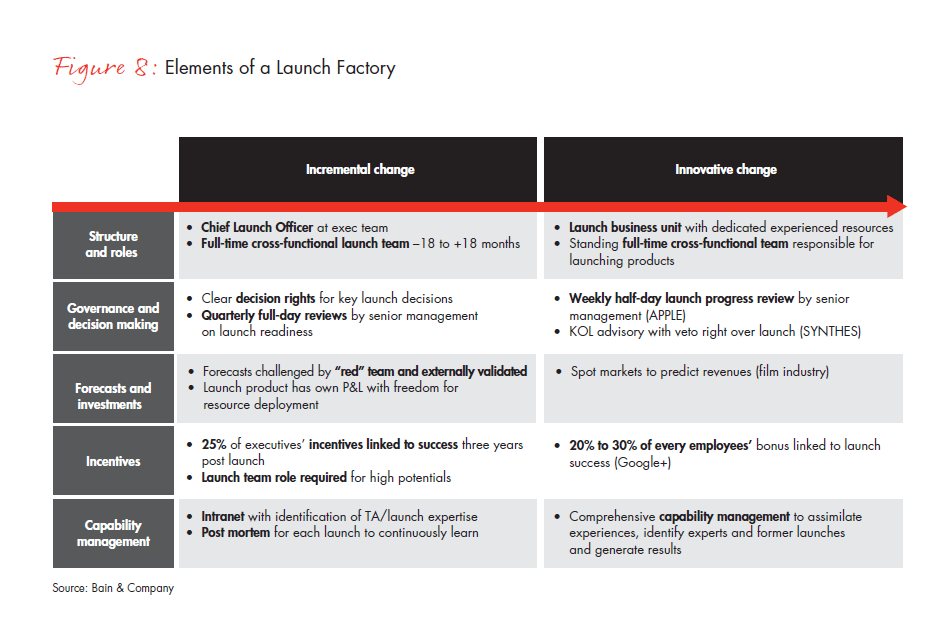
Companies that want a bolder approach to structure and roles might begin by establishing a chief launch officer at the executive team level, aided by a full-time, cross-functional team that operates 18 months before and after launch. Establishing a dedicated business unit or creating a standing full-time, cross-functional team to oversee all launches would be an even more innovative approach. More centralized launch functions also may require companies to reevaluate the role of brand teams. In the future, brand teams may need to be over-resourced around the launch—from one year before to two years after—and have a combination of traditional therapeutic area-focused marketers as well as launch experts. For brands further along the life cycle, these resources can be ratcheted back significantly.
The decision-making process can move ahead more effectively with clear decision rights and timelines, along with quarterly full-day reviews by senior management on launch readiness, instead of only one or two senior management reviews during the entire launch process. Adding even more frequent progress review meetings can avoid problems that might emerge if the process is done only quarterly. One healthcare company has even given KOLs “veto rights” over the development and launch approach.
Financial incentives tend to influence less than 10% of overall executive bonuses. Providing a quarter of executives with incentives linked to success three years postlaunch can produce a more highly committed team, but linking a third of every employee’s bonus to launch success would be even more innovative.
Instead of a routine checklist approach, a capability management system can help to identify people with the right expertise, along with a post-mortem approach to learn from previous launches and to continuously apply that learning.
The level of change any company can realistically implement will depend on its number of launches and consistency in launching products to date. Not every company will be able to execute innovative changes at every point, but moving beyond the typical approach can produce measurable results. For example, Biogen Idec established a dedicated launch-excellence function with experienced resources and a standing full-time, cross-functional team responsible for supporting all launch products, where a more typical approach might have been to simply set up a center of excellence.
As disruptive as these changes may seem, they are not unique to the pharma industry. Companies in other industries have used the Launch Factory approach to gear their organizations toward delivering continuous product launches (e.g., high-tech companies like Apple and Samsung or medical technology companies like hearing aid makers). Some lessons learned that produced commercial momentum include establishment of a launch business unit where all new products are grown until they reach a certain size; weekly management meetings to review launch progress (e.g., Apple); 20% to 30% of every employee’s incentive linked to the success of critical launches (e.g., Google+); data systems to predict uptake of new products (spot markets to assess launch success); and systematic post-mortem analytics to understand what worked and what did not (e.g., the film industry).
As the pharma industry adjusts to the new reality for successful product launches, leading companies that follow three practical insights will surge ahead:
- Shift from a one-size-fits-all to a tailored launch approach.
- Update the launch toolkit to clearly assess value, product profile, service and mobilization by market.
- Build a Launch Factory that captures learning and applies it in a repeatable process across all product launches.
Are you ready?
Michael Kunst is a Bain partner based in Boston and Munich. Rafael Natanek is a principal based in Bain’s London office. Loic Plantevin is a Bain partner based in Paris. George Eliades is a Bain partner in the San Francisco office. All are members of Bain’s Global Healthcare practice. The authors would like to recognize Annabell Geidner and Nicolas Stephan, both with Bain & Company in Munich, for their contributions to this Bain Brief.







