Etude
If you run a branded pharmaceutical company, you’re already living with the tension of balancing some reasonably good long-term prospects against a tough challenge in the next three to five years. Looking toward the end of the decade, there’s plenty to be optimistic about, as aging populations create growing customer needs, and advances in science and technology produce an increasing stream of innovation. But the pressure to deliver returns won’t get any easier in the short term, as CEOs navigate through rough waters caused by the loss of exclusivity from key in-line products, thin late-stage pipelines, regulatory agencies that are becoming more risk averse, eroding market access, declining reimbursement and commercial models that have lost momentum.
Bain & Company analysis indicates that big pharma revenues will be essentially flat over the next five years, with potential for some recovery later in the decade. By contrast, company projections and analyst forecasts predict growth for the top 10 branded pharmaceutical firms—some by as much as 5% to 7% over the same period. As a result, up to $20 billion to $30 billion in annual revenues may not materialize in 2016, with most of that directly falling to the bottom line. The implications for the CEO balancing act are large. The shortfall raises the stakes even higher for CEOs aiming to meet investor expectations in the near and medium term.
Taking a closer look, consider the way that two of the most daunting obstacles—pressure on pricing and restricted access to certain products—create headwinds to growth. We expect that pricing pressure will intensify over the next four years as many major therapeutic drug classes that are currently facing “medium” pricing pressure move toward the “high” pricing pressure area (see Figure 1).
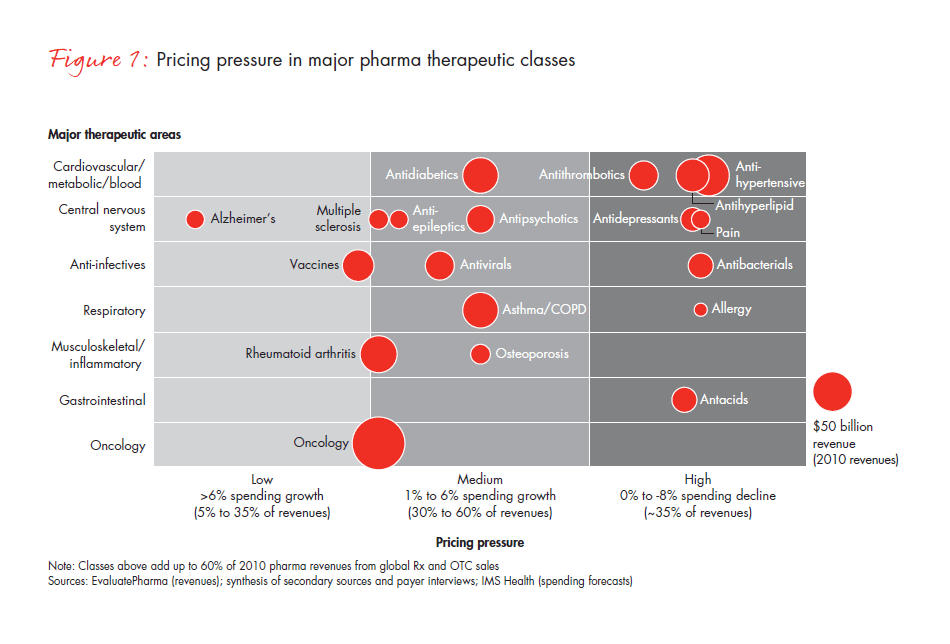
In addition to pricing pressure, more US public and private payers are restricting access to certain drugs, placing them on tiers that require high co-insurance and prior physician authorization, negotiations for lower reimbursement levels—or they simply deny access altogether. This dynamic is manifest in the case of Avastin. The US Food and Drug Administration has recommended the removal of the breast cancer indication from Avastin’s label. And in the UK, while it is licensed and legal, the National Institute for Health and Clinical Excellence (NICE) has determined that the drug will not be covered by the National Health Service for publicly funded patients because it is not considered to be cost-effective. Patients and their physicians in the UK must seek coverage through private insurance or apply as an “exceptional case” to the government’s Cancer Drugs Fund, which will end in 2014.
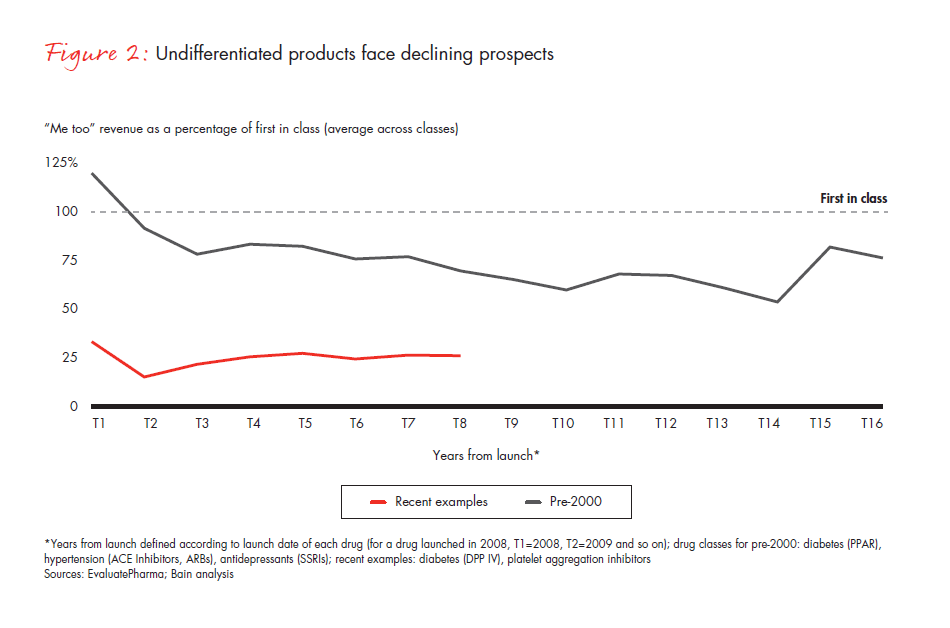
For products with low unmet need and high generics penetration, such as antihypertensive drugs, the combined effect of these factors is particularly strong. New launches are underperforming, compared with the past—particularly for less differentiated products (see Figure 2).
A portfolio of strategic bets
To beat the average and deliver on investor expectations of 5% to 10% shareholder returns in the next few years, leadership teams are pursuing strategies that include redesigning research and development (R&D) models, generating improved data to justify the use of their products and deploying new commercial models to align with the evolving buying pattern for ethical drugs. These approaches are all reasonable strategic bets, and the probabilities of success and timing of impact on the business will vary. However, there is another strategy that provides a low-risk complement to the portfolio of strategic bets: driving sustained and meaningful cost reduction and ongoing productivity improvement throughout the organization.
For most branded pharmaceutical companies, performance improvement has not historically been a central element of their tool kit. As long as margins remained sufficiently strong to support higher-cost operating models, branded pharmaceutical companies have deferred taking comprehensive actions to transform their costs. The typical approach to cost management has been episodic and incremental—closing R&D sites, consolidating manufacturing plants or reducing commercial investment for brands nearing loss of exclusivity. As CEOs strive for sustained cost transformation, many face an even larger barrier: cultural resistance within their organizations, where insularity can create a powerful impediment to change.
The difficulty of instilling a cost-focused culture has put many pharmaceutical companies at a disadvantage. A metric that is commonly used across industries—total per-unit non-R&D costs, that is, accounting for all manufacturing, sales and marketing, and general and administrative (G&A) expenses, and dividing them by the total units sold—can be applied to the pharmaceutical industry to understand productivity trends over time (see Figure 3). Compared with the productivity gains we would expect from comparably mature industries, the pharmaceutical cost gap amounts to 25% to 30% over this time frame alone (see Figure 4). In most industries, companies realize continuous productivity gains across functional areas, helping to offset inflation and produce value for customers. While some of the cost increases in the largest pharmaceutical companies are due to exogenous factors such as regulatory requirements, in our view, these factors fail to explain the wide productivity gap. Even the generics industry held per-unit costs flat during this same time frame, despite being exposed to many of the same factors.
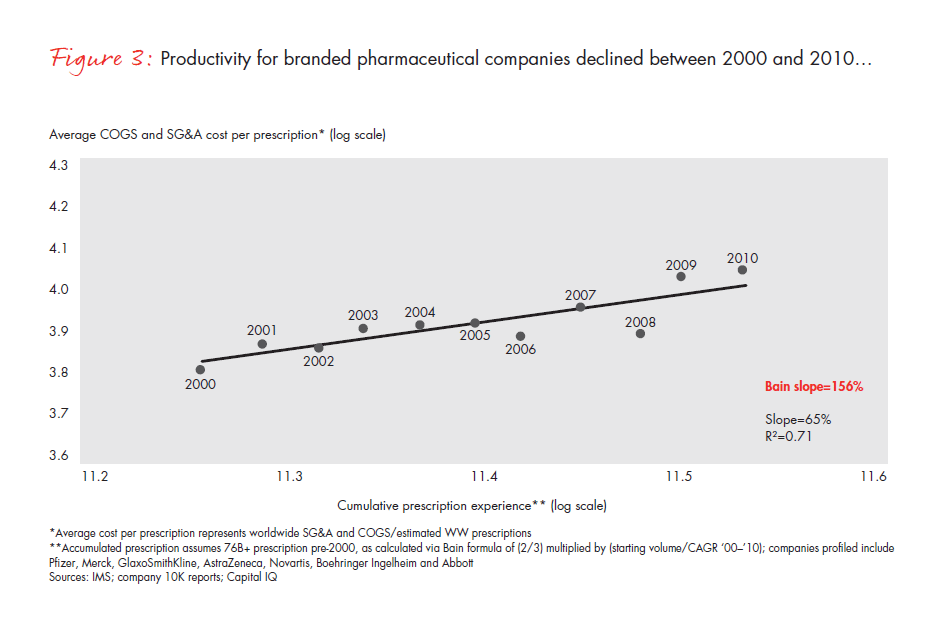
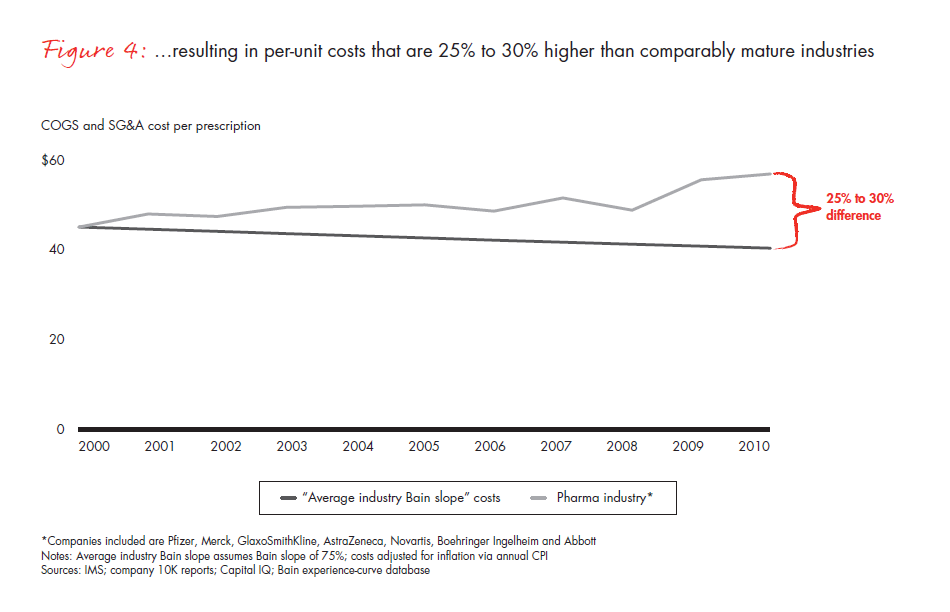
As difficult as the next five years will be, the opportunities for performance improvement are significant and within reach, making this a valuable component of the portfolio of strategic choices. If pharmaceutical companies can achieve even 60% of the productivity gains that comparably mature industries will deliver over the next five years, they will eliminate virtually all of the projected profit declines they now confront.
Guiding principles of cost transformation
Most pharmaceutical companies recognize the need for fundamental cost transformation. The good news is that there is a wide range of potential actions that companies can take to change their cost structure fundamentally. Of course, the applicability of these approaches depends on a specific company’s situation. And in some cases, companies may not know the right way to approach this transformation. For example, you may ask an organization about implementing such levers, and the response may be “we’ve done that,” but the reality is that these actions may have been applied selectively or inconsistently, piloted and then not fully implemented, and most of all, not pushed to sufficiently ambitious levels of change. In addition, some cost areas are considered “nonnegotiables,” when they shouldn’t be, and thus, the right questions are never asked.
A good example of that lies in the commercial side of the business. Many companies have begun to shrink their commercial resources in the face of patent expiries and have made mix adjustments to sales and marketing spending (such as greater use of digital channels or less personal selling) for the remaining products— without producing any substantive change to the selling model itself. Few companies, if any, have created a commercial model designed for the current and evolving market based on maximizing the absolute return on investments of the resources used (such as market research, market access, government affairs, account management, salesforces, direct-to-consumer promotion and so on) and the markets in which they are deployed.
Cost-reduction efforts can become stuck in distinct silos within the organization, but it takes a comprehensive approach to transform the cost structure. Some guiding principles can help.
- Everything on the table. The entire cost structure must be part of the scope. No exceptions, period.
- Facts first. Conduct a fact-based review of all activities, both direct and supportive, to evaluate their true clinical and bottom-line impact. For example, what marketing research and analytics or internal financial planning and analysis reports are “nice to have” and not “need to have”? What is the true return on investment for the broad range of commercial support functions?
- Outside in. Compare your cost management to best in class, both inside and (more important) outside the pharmaceutical industry.
- The road ahead, not the rearview mirror. Set targets based on a realistic, multilevel view of where the industry and the business will be in three to five years.
- Justify what’s needed, not what’s already there. Zero-based budgeting builds up from zero what costs are necessary to execute a given activity or function, as opposed to anchoring in current spending levels and looking for reduction opportunities.
- Banish the lore. Don’t accept the rationale that “we tried that and it didn’t work.” More often than not, it has not been tried in the right way and with full effort. In addition, it may have been tried under different circumstances. Recognize and reward bold thinking that challenges the accepted wisdom of what may work.
How the leaders do it
Analogous industries that have undergone significant, systemic change and successfully managed this type of transformation in the face of shrinking profitability can provide valuable lessons for the pharmaceutical industry.
Over the past few years, medical technology companies have faced challenges that are similar to the branded pharmaceutical industry, and some of them have begun to act on the cost imperative. Among the challenges: increasing competition from me-too products because of a lack of perceived differentiation in branded devices; the lessening of physician discretion in product choice; professionalization of the materials management functions, creating greater efficiency and reduction in costs; and increasing consumer engagement in decisions about all aspects of their healthcare.
One medical device company faced many of the same challenges global pharmaceutical companies face today—a complex organizational model, little experience in cost reduction and cultural resistance to organizational change. When the company was confronted with the need to change, its initial response was, “We are a growth and innovation company,” implying that cost reduction was not necessary. But the CFO was clear about why the firm had to take action. He said, “We got drunk on our blockbuster’s profit and spent like drunken sailors. Now it’s time to sober up.” He and the CEO had both worked in other industries, so they were not caught up in a rigid “medtech” point of view. They understood that cost reduction requires absolute commitment from the top—and that it must be among a company’s top three priorities. Acknowledging the imperative to change required taking the following actions: (1) publicly declaring their goals to the street; (2) holding senior executives accountable for a focused set of high-value business objectives, tying career progression and incentives to the plan; and (3) setting up a parallel management organization to manage the take-out (such as a program office) with weekly updates to senior management. When restructuring jobs, the CEO and CFO took the mindset of the tens of thousands of jobs they would preserve by taking this strong action.
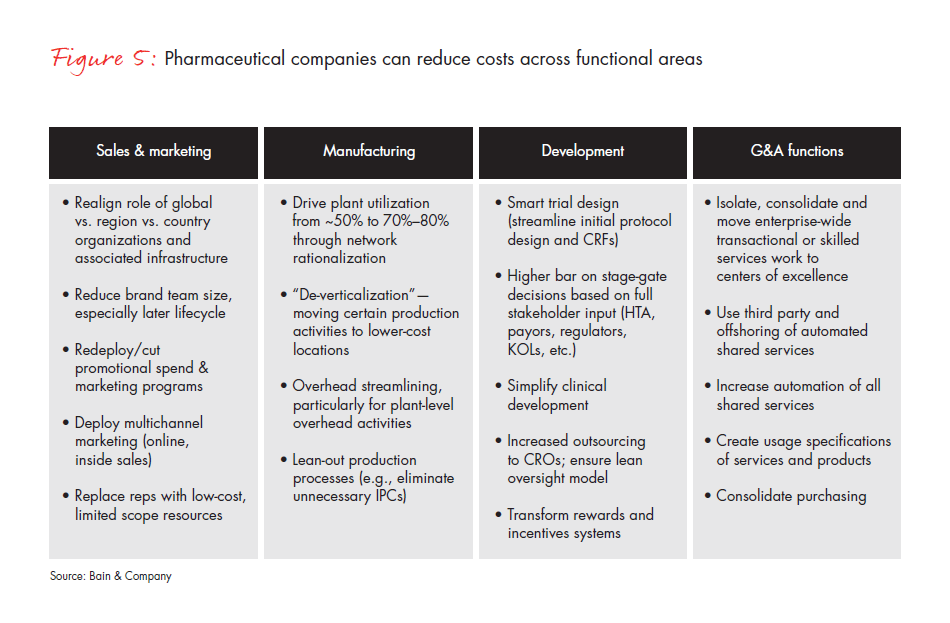
Because of its willingness to commit to change, this medical device company was able to reduce a quarter of its spending through a few major initiatives: It first redesigned its commercial model for the largest business unit to serve purchasing stakeholders more efficiently and effectively. The company then redesigned IT and quality functions from a “blank sheet of paper” using zero-based budgeting (see Figure 5). It focused on additional savings in R&D, regulatory, finance, legal, HR, facilities and indirect spending. Finally, it conducted an independent “private equity cost view” to challenge the organization by bringing in outside experts to give its cost structure a fresh look. The company also set up the organization to eliminate costs continually from the system through lean business processes and ongoing restructuring. As a result, the company institutionalized multiyear cost reduction, making it the “new normal.” Accordingly, the board of directors approved a plan to reduce approximately $1 billion of addressable savings on a base of $4.2 billion.
Although it might seem counterintuitive, the defense industry also illustrates some of the challenges and strategic cost transformation imperatives facing large pharmaceutical companies today. Like the pharmaceutical industry, the defense industry is driven by R&D, with a long product development life cycle and significant barriers to entry. The defense companies had a proliferation of product lines, similar to pharmaceutical companies, whose products are split across numerous therapeutic areas. Because the public sector was a major customer for defense companies, their spending was also driven by the public sector; in the pharmaceutical industry, government funding provides access to ethical drugs for most patients in developed markets. Finally, the defense industry lived with a “cost plus” mentality—it certainly was not a cost-focused sector historically—as does the pharmaceutical industry.
After a period of growth in the early 1980s, the US defense industry faced large and sustained cuts in spending over the next two decades. When the US Department of Defense cut its spending by more than 30% in the early 1990s, individual defense companies began to struggle. Their large R&D investments failed to pay off. Their bloated cost structures were simply not in line with the new reality.
While rivals failed, General Dynamics emerged as a winner. How? General Dynamics did four things right: It accepted the new reality, narrowed its core to focus on areas with a path to profitable economics, undertook aggressive strategic cost management and acted boldly to pursue subsequent growth in adjacent markets. General Dynamics created significant value employing this strategy, while others suffered. Even though the business shrank from $10 billion in 1990 to $3 billion in 1993, profitability improved from a negative 8% to a positive 10%. Pursuing other attractive markets subsequently helped General Dynamics grow to a highly profitable $30 billion company by 2010, and it continues to perform well relative to its competitors under difficult conditions.
Achieving sustainable cost improvement
Successfully reducing cost and improving organizational performance requires more than the usual incremental approach to change. Even for firms that know what needs to be done, mobilizing the organization and changing its culture remains a major challenge. For branded pharmaceutical companies, it is also important to preserve the elements of the organization that are critical to foster creativity and innovation. That not only increases the odds of success for the reshaped company, but it is also critical in gaining the acceptance of change within the organization.
In our experience with the pharmaceutical industry and other industries facing equivalent pressure, we have identified five critical elements to achieving sustainable results, which, while self-evident, require significant thought and attention right from the start.
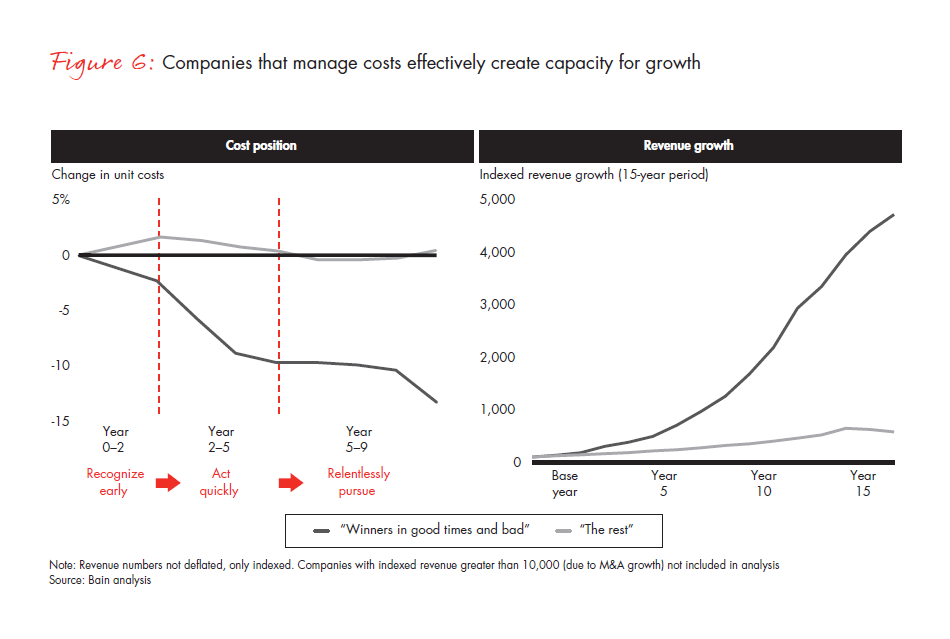
1. Articulate a clear and compelling vision. Understand where you are headed and why sustained cost transformation will promote success, but be realistic (see Figure 6). Senior-level sponsors need to be committed to the vision, understand how disruptive the change will be and balance the vision with the organization’s capacity to absorb change. For branded pharmaceutical companies, it is critically important to preserve the elements of the organization that create and foster creativity and innovation in order for change to be accepted.
2. Mobilize leaders. Clearly define change roles (starting at the top of the organization), and then identify and engage sponsors of this change across organizational layers—what we call the “sponsorship spine.” The truth is that change depends on the effectiveness of those providing the consequences—the sponsors of the change.
3. Change behaviors. Identify the few critical behaviors required to produce results, then shift and reinforce behaviors by changing consequences and measuring progress. To change behavior, you will need to change consequences first, and this starts at the top with your leadership team.
4. Set up a parallel management structure to manage change. Reduce your time to realization with a decision cadence that plans, tracks and measures progress and mitigates ongoing implementation risks. Having a program office that tracks progress across the organization is imperative to achieving goals.
5. Embed the change. Create a new source of sustainable competitive advantage by investing in capabilities that build a repeatable model for change. Build a culture of continual improvement that encourages employees to think constantly about how they can perform their jobs more efficiently and effectively, and then reward them for doing so.
Executing these actions requires strong commitment from the front lines to the leadership team. But cost transformation offers high returns with a relatively low risk profile and provides the necessary foundation for today’s branded pharmaceutical companies to lead the industry as future growth opportunities take hold.
Andy Pasternak is a partner with Bain & Company and is based in Chicago. Maria Gordian is a Bain partner based in New York. Both are senior members of Bain’s Global Healthcare practice. Paul Cichocki is a Bain partner based in Boston and the leader of Bain’s Americas Performance Improvement practice.





