Global Healthcare Private Equity Report

At a Glance
- Biopharma and life sciences tools are attractive: Within these two subsectors, more than 600 healthcare buyout deals have been executed over the past five years globally, fueled by strong end-market growth tailwinds, recession resistance, and attractive returns for private equity. Given that these trends are expected to continue, executing coherent deal strategy in biopharma and life sciences tools has become a must-have for US/EU funds.
- Subsectors within life sciences matter: Out of the roughly 410 US/EU biopharma and life sciences tools buyouts executed over the past five-and-a-half years, more than 80% have been in eight subsectors, each with a different set of dynamics.
- Concentrated dealmakers, long tail of participants: About 65% of biopharma and life sciences tools deals in the US and Europe were conducted by around 50 funds with a large amount of midmarket participation.
- Lessons learned from dealmakers: Funds that have a repeatable approach to closing deals have a very sharp perspective on “where to play” and “how to win” and have flexed the type of deal they are prepared to do to be successful. Differentiation in subsectors is increasingly driven by the quality of science and depth of expertise in a specific disease or technology.
This article is part of Bain's 2023 Global Healthcare Private Equity and M&A Report.
The life sciences deal market—that is, marketed biopharma products and the ecosystem of companies supporting their research, development, and commercialization—has been an attractive place for private equity (PE) to put capital to work. These markets—which include life sciences tools (LSTs), diagnostics (Dx), lab services, outsourced biopharma services, and pharma software/IT—are historically recession resistant and have all been driven by strong end-market growth tailwinds. These sectors have also seen strong returns within healthcare, which itself is an attractive industry vertical.
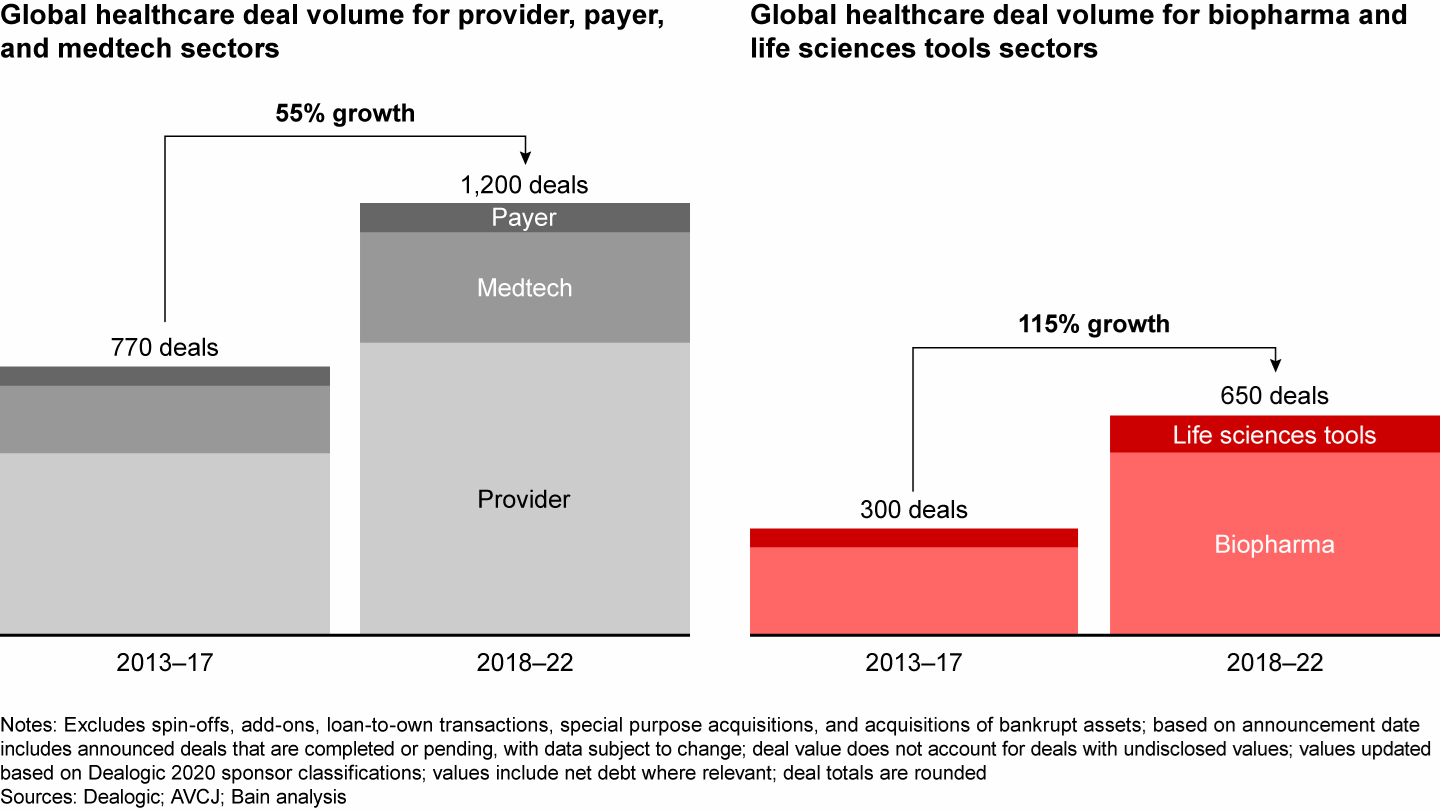
Given all of these dynamics, the volume of PE dealmaking has increased significantly in this area, and it has become a must-win sector for funds. Deal volume in the past five years in biopharma and life sciences tools has increased 115% compared with the previous five-year period (see Figure 1). Payer, provider, and medtech deal activity increased only 55% across those same two time periods. We count 650 unique buyout deals globally for life sciences assets completed since 2018 (excluding preclinical and clinical stage therapeutic investments given their risk/return profile).
Deal growth in the life sciences sector outpaced other sectors over the past decade

The internal rate of return (IRR) for life sciences deals over the past 10 years has been around 25%, with the top quartile achieving more than 50% IRR across biopharma products and services and life sciences tools and services. The variance in returns in life sciences sectors is also wider than other healthcare investment areas, highlighting the effects of technology and regulatory risk embedded in these investments and the value of specialization in mitigating those risks. These deals tend to be earlier in the life cycle of an asset, and therefore bear a spikier risk/return profile.
Placing the right bets: Subsectors within biopharma and life sciences tools matter
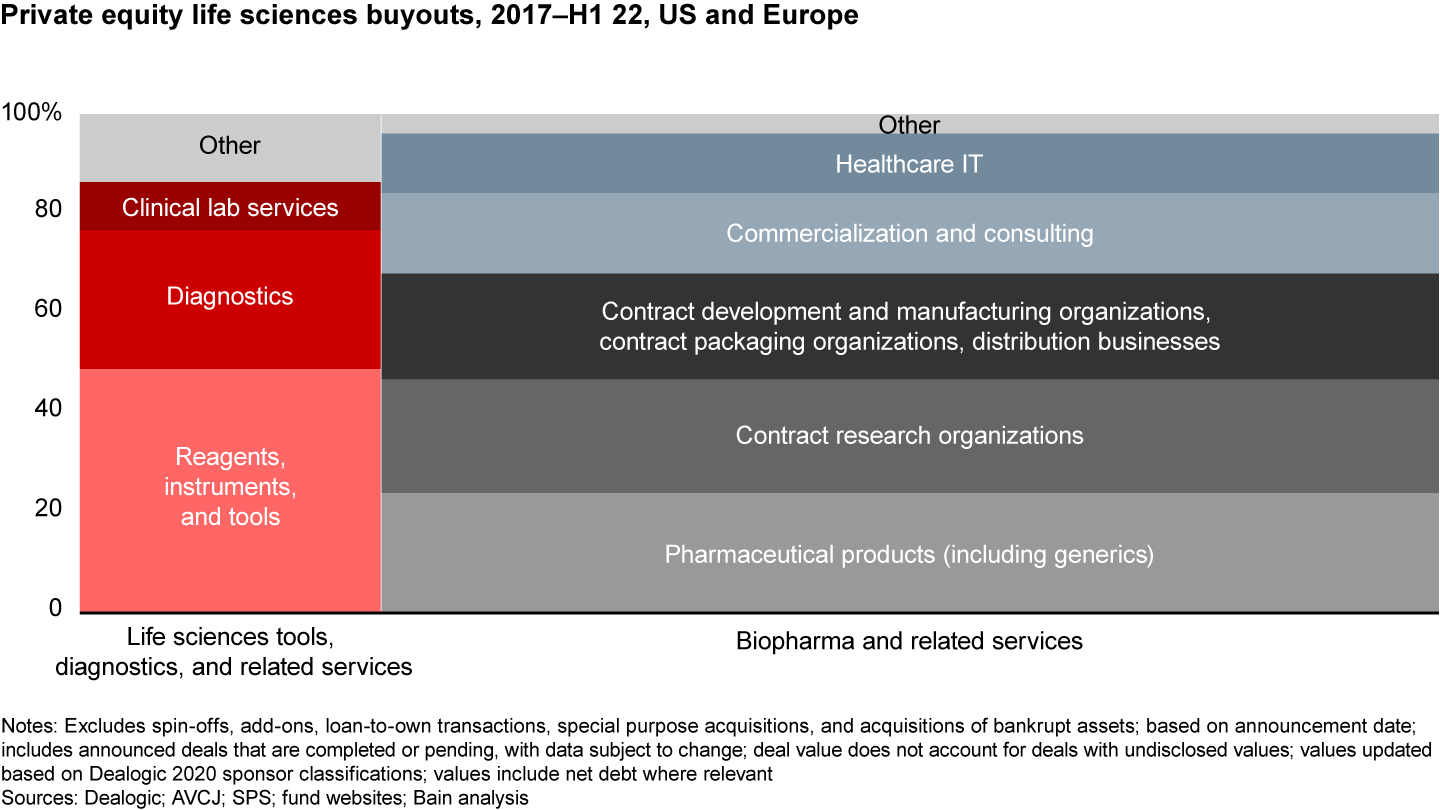
A sector-by-sector analysis of the deals done in the US and Europe reveals that eight subsectors represent the vast majority (85%–90%) of the deals done within private equity (see Figure 2). Given that each sector has very different business models, sets of assets, and winning investment theses, it is our strong belief that funds need to develop a custom view on which assets they are going to “hunt” for.
Out of the roughly 410 unique life sciences deals completed for US/EU assets between 2017 and mid-2022, 75%–80% were pharma-related; the remaining 20%–25% were in life sciences tools or diagnostics. Within biopharma deals, there were five major sectors of activity that each represented at least 25 deals—contract research organizations (CROs); contract development and manufacturing company (CDMOs)/contract packing organizations/distribution businesses; commercial pharma products; commercialization services; and pharma IT. Within life sciences tools, two major sectors of activity represented at least 25 deals—reagents/instruments/tools and diagnostics. Clinical lab services represented a smaller number of deals completed but were mostly all in the EU.
The overwhelming majority of life sciences buyout activity occurred in eight categories
Life sciences landscape: Despite upside, biopharma and life sciences tools remain fragmented, challenging spaces to play
Private equity buyout participation in biopharma and the life sciences tools subsector is widespread, with around 200 funds participating in around 410 unique deals. However, there is a concentration of high-velocity PE dealmakers who are responsible for most of the activity. A large fraction of dealmakers are the midmarket life sciences specialists, although some large-cap funds have been high-velocity dealmakers as well.
There were roughly 460 PE transactions for US- and European-based biopharma and life sciences tools assets between 2017 and the end of the first half of 2022. Roughly 410 were “unique” deals, and roughly 50 deals were multifund. In total, around 200 private equity firms participated in at least one life sciences deal, and around 170 of those made one to two investments during that time.
The number of unique funds participating in a life sciences deal increased from about 45 in 2017 to around 80 in 2021, reflecting not only the presence of more “hunters,” but also the increased productivity/activity of funds focusing on life sciences as a sector. Some 45 to 50 private equity firms were responsible for 290 deals (65%) of those closed (at least three-plus closed deals).
Midmarket PE funds with a life sciences focus represent 40%–50% of the top 50 dealmakers (which include Arsenal Capital Partners, Ampersand Capital Partners, GHO, ArchiMed). Some large-cap PE funds each closed four-plus life sciences deals—Carlyle, EQT, Astorg, GTCR, Nordic Capital, Advent, Blackstone, and KKR.
As the level of interest in biopharma and life sciences tools investments has increased, we have also observed changes in the deal processes—exacerbated by the boom in post-Covid dealmaking—and changes in the underlying nature of the businesses being acquired that have created additional complications for potential investors:
Auction processes: Sellers used auction processes with larger pools of bidders, shortened bid periods, and limited access to management data to drive more aggressive valuations and outcomes.
Competition with strategics: Rapid maturation and inflection points in demand related to areas such as cell and gene therapy created intense interest from strategic buyers for smaller assets that historically have traded sponsor to sponsor, driving multiples higher and creating a scarcity of available assets:
- This phenomenon was acutely felt at the intersection of LSTs and manufacturing, with Thermo Fisher Scientific and Catalent acquiring viral vector CDMOs Brammer Bio and Paragon Bioservices, respectively, and companies like Thermo Fisher, Sartorius, and Cytiva (Danaher) aggressively acquiring bioprocessing specialists with unique offerings for cell and gene therapy applications such as PeproTech (cytokines for research and good manufacturing practices) and BIA Separations (viral vector-focused chromatography technology).
Diligence complexity: Differentiation in subsectors is increasingly driven by the quality of science and depth of expertise in a specific disease or technology, and thus more challenging for many investors to evaluate.
Together, these challenges have raised the bar for private equity firms to execute successful investments within the biopharma and life sciences tools landscape. Funds that have closed five or more deals in biopharma and life sciences tools often have focused teams, great relationships with venture capital and small-cap-focused feeder funds, and a willingness to participate in more deals at smaller ticket sizes with a higher risk profile.
Lessons learned from dealmakers: Sponsors can mitigate the challenges within biopharma and life sciences
Analysis of life sciences deal activity reveals the ways in which dealmakers have been thoughtful about where to play and how to win. They have deployed a set of strategies to overcome some of the main challenges we see in life sciences deal participation for private equity:
Networking: Build and utilize a wide network of outside advisers with scientific and commercial acumen to proactively vet scientific trends, identify subsectors of focus and gem assets within those areas, and prioritize deal strategies and value creation opportunities early in the investment process.
Talent strategy: Deal teams with scientific backgrounds (PhDs, MDs) focused on the life sciences sector—and sometimes subsectors within life sciences—can overcome scientific, technical, and regulatory expertise hurdles needed to build conviction on a theme and target early in a process (or preprocess) and effectively prewire and manage challenging dynamics with generalist investment committees to ensure competitive positioning in processes.
Portfolio effects: Dealmakers often double down and make multiple investments within a sector and across subsectors to leverage shared, collective expertise and employ pattern recognition to create value across their platforms:
- Arsenal Capital has built scale platforms across multiple areas of the outsourced services market (WCG in institutional review board and clinical trial support; Certara in software-aided drug discovery; CellCarta in biomarker and related lab services; Lumanity in commercialization).
- GHO has made a series of separate investments in a range of distinctive CDMOs (such as Ardena, FairJourney Biologics, RoslinCT, and Sanner).
Risk profile: While the risk profile of preclinical/clinical stage assets is addressable only for a subset of PE funds, some large-cap funds have been building out capabilities to support investment in this area.
Flexibility: Given vendor fragmentation, scale asset scarcity, and rich valuation multiples on category leaders, dealmakers have gotten creative in their structures to access a wider range of deals. Examples include:
- Platform building: Behrman Capital used tuck-in acquisitions to help its CRO Emmes build a set of unique capabilities that helped transform the business from a government-focused CRO to a specialty CRO serving industry sponsors.
- Public-to-private: Gurnet Point Capital and Patient Square Capital took Radius Health private to invigorate the trajectory of its commercial-stage osteoporosis asset, Tymlos, while sharpening the focus of its pipeline and reducing noncore development costs.
- Technology/growth bets: KKR has acquired through its Gamma Biosciences platform five earlier-stage companies that provide a variety of innovative biomanufacturing materials or equipment utilized in manufacturing of advanced therapeutics like cell and gene therapies.
Forging a path forward for private equity: Sponsors will need to define their unique strategies
We continue to believe that life sciences will be a long-term attractive place for private equity to participate, although there may be some nearer-term noise given the factors currently impacting the economy and the biotech funding-cycle dynamics. That said, it is not an easy place for funds to navigate successfully. We believe that funds have to wrestle with a number of questions to define their strategy:
- What is our participation model in life sciences deals?
- Are we looking for only the premium, category leader assets? Are we prepared to dig deep to meet valuation expectations? Do we know, or are we tracking, the assets that are likely to come to market in the next few years? Are we developing unique angles around the deal thesis to invigorate the next wave of growth?
- Are we going to compete with life sciences specialists and invest early in technology/growth/platform bets? If yes, what’s our strategy to build our internal competencies and processes, along with external advisory teams? Are we going to use innovative deal strategies to put money to work?
- Which of the life sciences subsectors are we participating in (and which are we not)?
- What are our unique investment thesis and connections in the space? How can we keep ahead of the competition as more funds enter the space? How can we avoid spending time on deals that are going to be fought over fiercely?
- Can we stretch the “type” of deal we do to cover a broader range of transactions?
- Would a public-private, or a carve-out, or a growth-equity investment fund ever be on the table? Would we ever stretch into biopharma therapeutics?
Given the opportunities for compelling returns within life sciences, the bar is high to win the right deals as competition for these assets holds strong and valuations have run up, even within the current macro context. Clearly defining a life sciences strategy—where a fund will choose to play and what gives that fund a right to win—will be critical, both in the face of a downturn and beyond.