Article

We have entered a “golden age” of innovation in clinical trial operations. New solutions are flooding the space to address the rapid growth in trials, intense competition for trial participants, slow and cumbersome protocols, myriad setbacks from the pandemic, and the promise of decentralized and digital trials. With this innovation comes significant complexity, but sponsors are in a unique position to mitigate it.
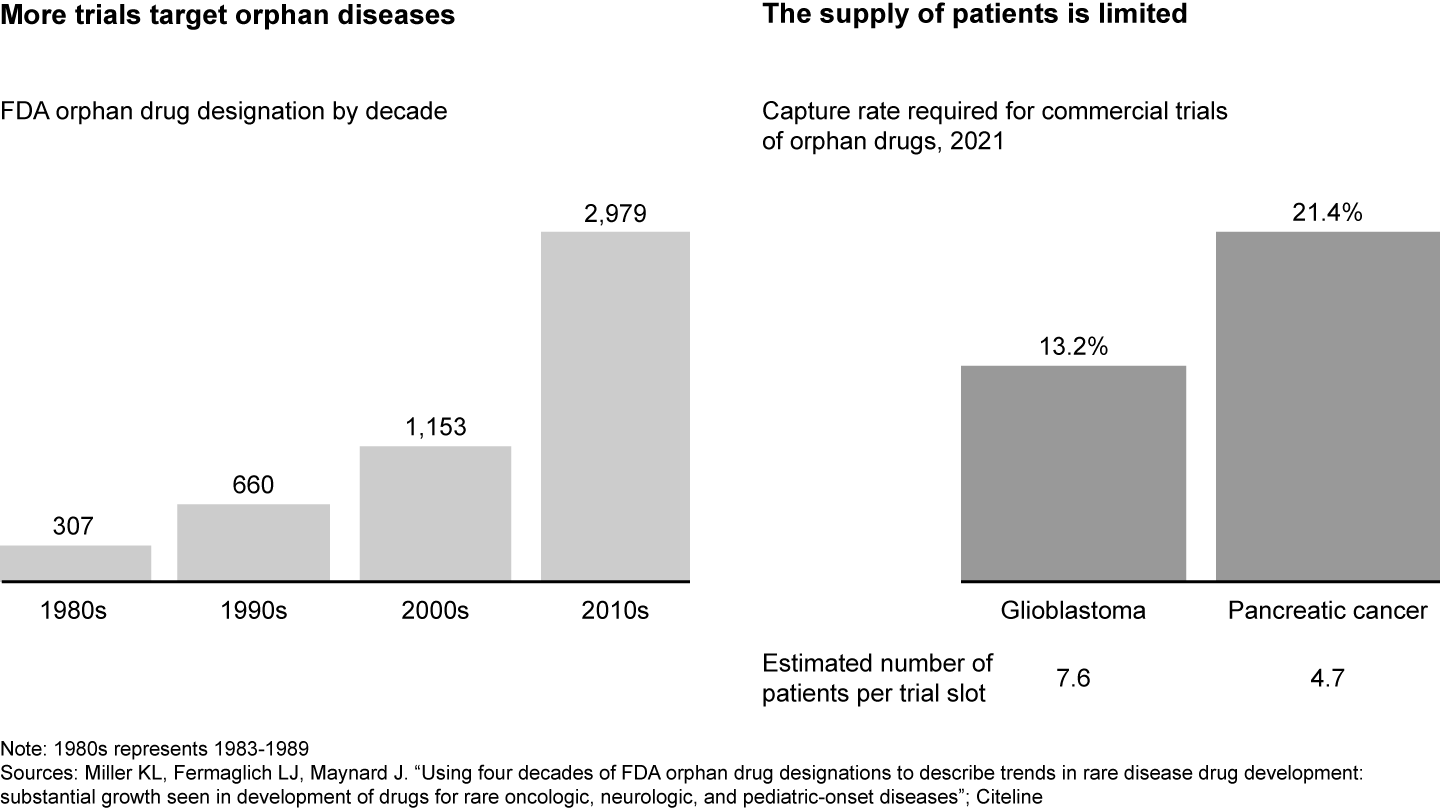
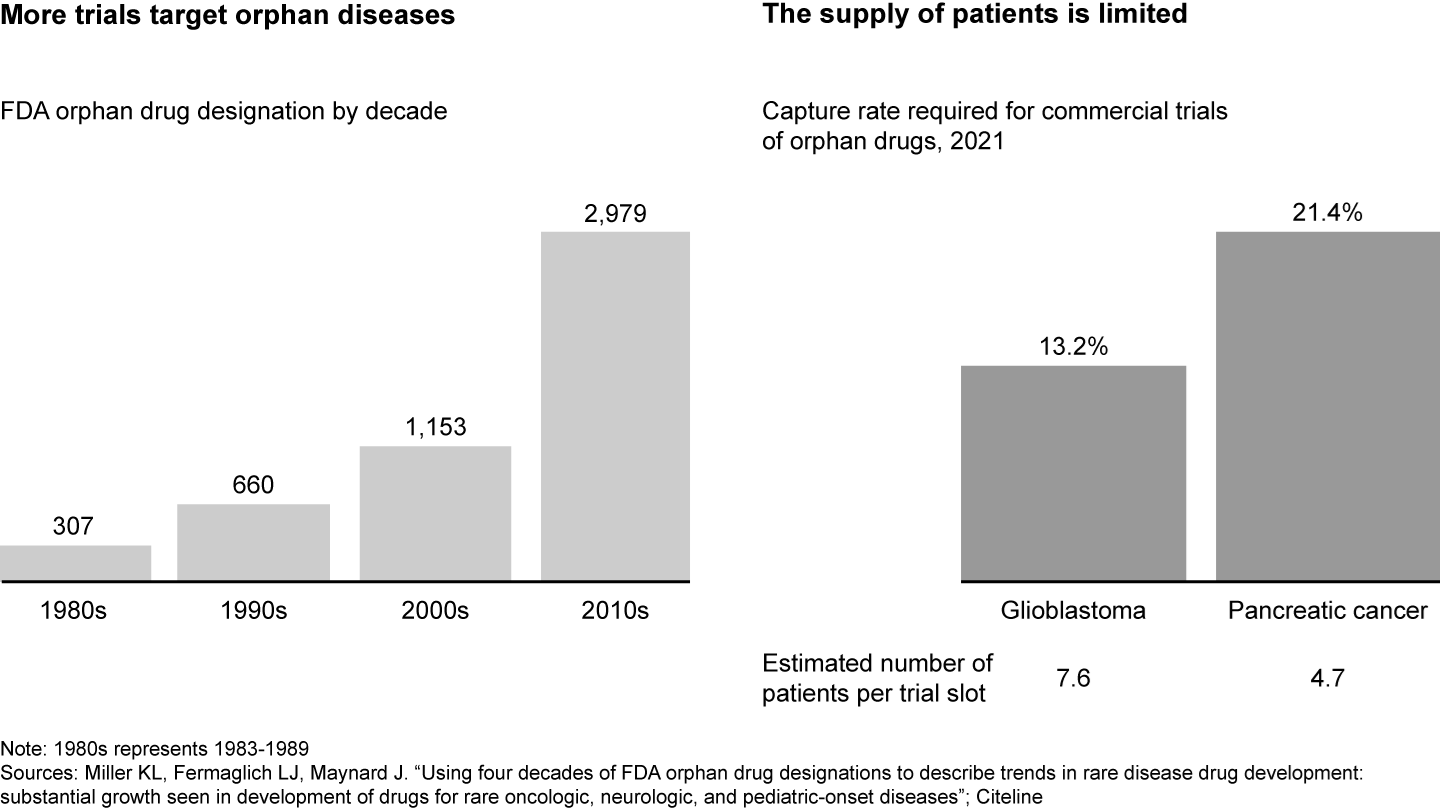
The clinical trial landscape is increasingly competitive (see Figure 1). While the number of industry-sponsored trials in the US has stagnated in the past decade, there has been an increased focus on rare disease or specific biomarkers with inherently smaller patient populations. For example, drugs that target orphan diseases, or those that affect fewer than 200,000 people in the US, have almost tripled between 2000 and 2020. As a result, there is fierce competition for an already limited pool of patients and sites. For instance, oncology trials for pancreatic cancer need to capture 21% of all patient cases in order to fill all trial slots, meaning there are only around five potential patients per trial slot.
And delays are becoming commonplace, especially as the Covid-19 pandemic and other geopolitical events have exacerbated the issue. These delays and disruptions do not bode well for pharma companies’ bottom line, as getting to market ahead of competitors provides tangible value.
We have seen an explosion of new tools, capabilities, and vendors that aim to harness digital capabilities and new methodologies to address these challenges and, ultimately, deliver better, faster trial outcomes. The e-clinical solutions market has grown around 17% per year since 2016. In the next five years, it is expected to double in size, becoming an $18 billion market.
While these digital tools have advantages, such as the enhanced ability to find new and more diverse trial participants, the emergence of this new ecosystem has also contributed to trial complexity. And it has not automatically translated to a better customer experience.
How can sponsors navigate this landscape of long delays, higher costs, tight resources, and new players? Leading sponsors are doubling down on the physician investigator experience—a powerful differentiator that can help recruit more patients and reduce delays in trials.
The customer experience imperative
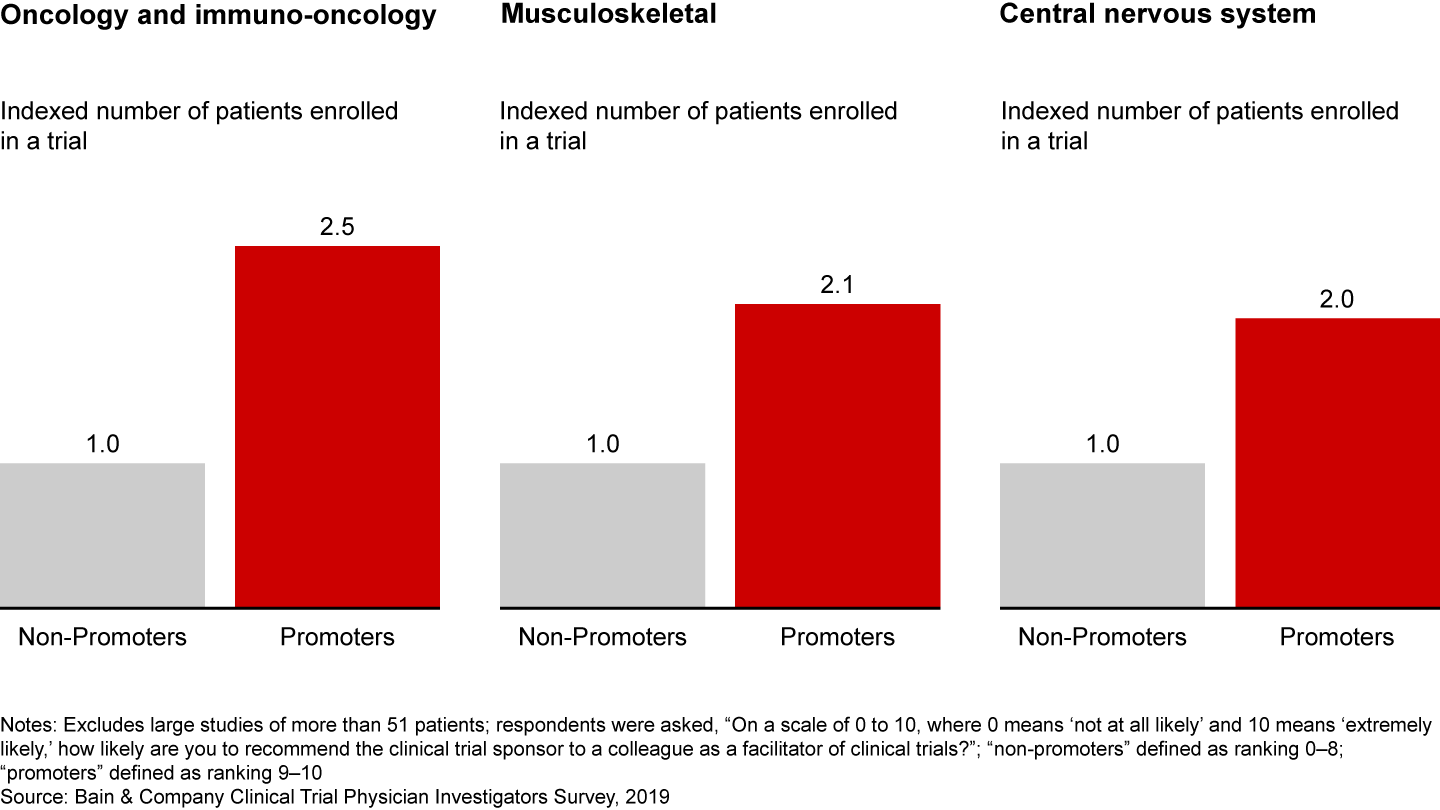
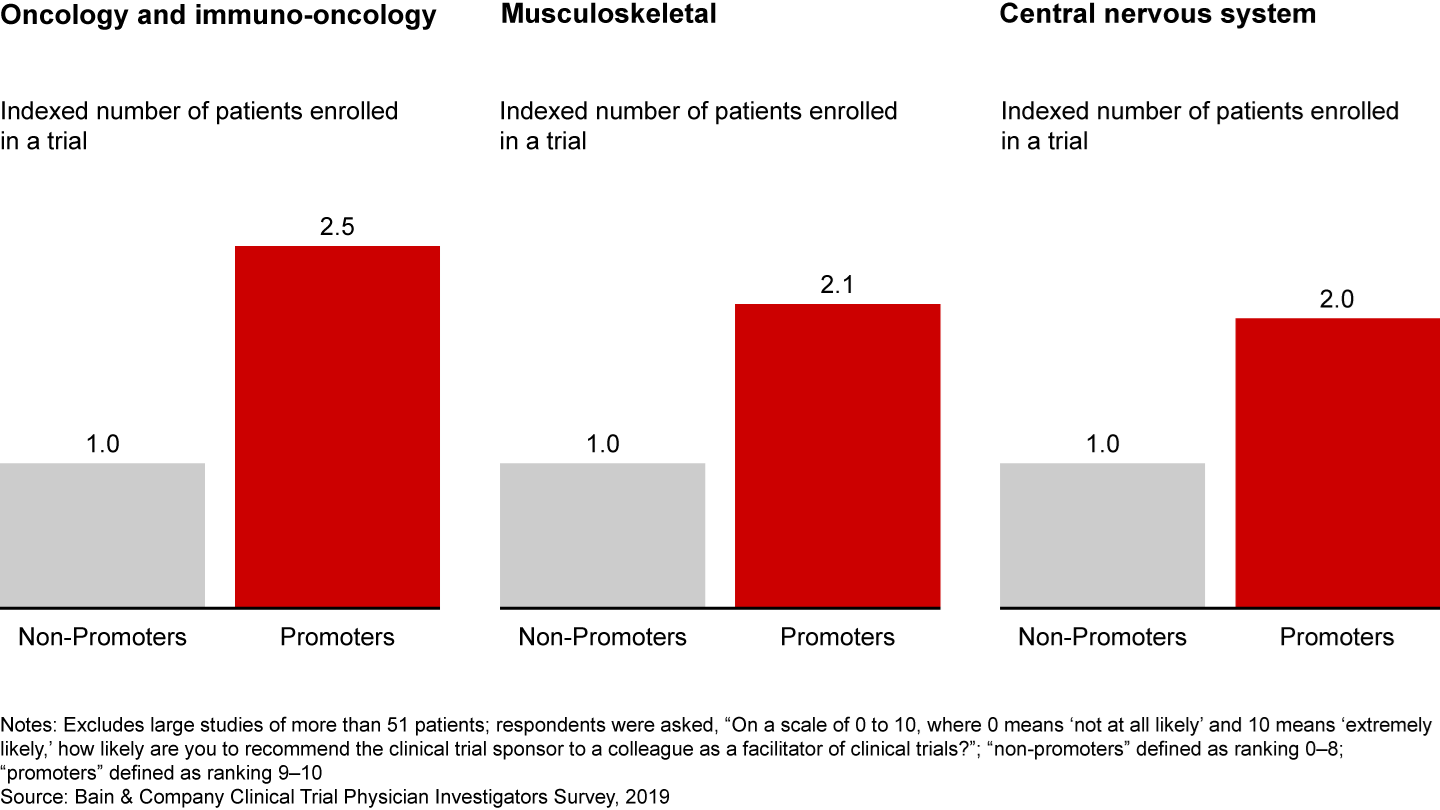
Bain research shows that a better investigator experience leads to loyalty and, ultimately, faster trial recruitment and completion. In fact, investigators who have a superior experience with a sponsor enroll twice as many patients (see Figure 2). This holds true across disease areas, from oncology to musculoskeletal to central nervous system.
A good customer experience is a foundational, yet often-overlooked, differentiator in today’s competitive environment. After all, investigators decide which trials to participate in based on factors that extend beyond potential therapeutic outcomes, like prior experience and sponsor reputation. Yet opportunities for improvement abound, as investigators, site administrators, and nurses continue to stress the same addressable pain points, across investigator sign-up, patient enrollment, and ongoing delivery and monitoring.
First, they feel burdened by administrative tasks, from long contract and budget negotiations to repetitive, cumbersome paperwork—especially today, given the added pressure of staffing shortages. In addition, site trainings can be impossibly long and dense. Busy staff members are often asked to read hundreds of pages or attend full-day sessions on systems they already know. Finally, investigators feel undervalued due to a lack of direct engagement with sponsors. Dissatisfied with transactional relationships with contract research organizations (CROs), investigators are hungry for more in-person interactions to discuss both scientific and operational topics.
Leading sponsors are listening. We have seen several sponsors start to implement best practices and improve the investigator experience. Here are a few of the approaches they have taken:
- Streamline systems. To combat redundancies and overwhelming workloads, some customer-experience leaders are integrating systems wherever possible and setting up online portals. For instance, Johnson & Johnson has reduced manual annotation and data entry through digital forms and centralized meta-data management systems, with the goal of implementing AI-enabled patient forms within the next year. As data solutions balloon to a $9 billion market in the next five years, leading sponsors will emphasize not just the customer experience but also the companies they trust to help them shape that experience.
- Take a customer-centric approach to site training and setup. Some leading sponsors are slimming down the multiday training regime to provide a simplified training document that is focused on “must know” items and supplemented with a more extensive document or website that investigators can reference as needed. In addition to these resources, many sponsors are heavily investing to provide trial site staff with access to 24/7 support via live chat, email, or phone.
- Increase in-person interactions. Some best-in-class sponsors are already meeting investigators’ desire for more communication. These companies start by engaging clinical teams during site and investigator selection to identify the most appropriate matches and ensure sponsor representatives show up on-site regularly during trials. For instance, for site identification, Biogen relies on a local network of medical science liaisons to identify key contacts at trial sites and facilitate introductions. These companies also connect investigators with the right people, such as contacts in clinical operations and clinical development, throughout the course of the trial. This direct contact can align investigators and sponsors on shared objectives and ensure trials do not stagnate.
- Strengthen CRO partnerships. Sponsors’ relationships with CROs can be highly transactional and lack clear communication. To ensure total alignment on trial goals, leading sponsors are building more innovative, collaborative, and mutually beneficial partnerships. There are several ways to do this, including establishing two-way transparency, two-way feedback loops, shared key metrics, and more.
Amid CRO involvement, increased decentralization, and a deluge of new tools and vendors, sponsors may not always feel as though they have full control over the investigator and patient experience. But tackling investigators’ pain points can be a foundational step in delivering an industry-leading trial experience, eventually separating the best from the rest.