論説

概要
- Rapid innovation has had the unintended consequence of burgeoning complexity for clinical trial sites, with 56% reporting that complexity has increased from three years ago, according to a Bain survey.
- Today, 53% of sites say they lack the bandwidth to run trials, up from 14% before the pandemic.
- Piecemeal trial solutions promise improvements that are misaligned with sites’ actual experience.
- With five principles, pharma companies can design next-gen trials that reduce delays, build competitive advantage, and bring critical innovation to patients.
A new drug’s mechanism-of-action exclusivity often lasts only half as long as in the early 2000s, fueling pharmaceutical stakeholders’ calls for accelerated trial timelines. Capture rates are now staggering, often representing 10% to 20% of all patients. At the same time, 53% of clinical trial sites say they have insufficient bandwidth to support their current phase 2 or 3 trials, according to a Bain survey. And 56% say complexity has increased from just three years ago (see Figure 1).
Clinical trials are facing an unsustainable confluence of challenges

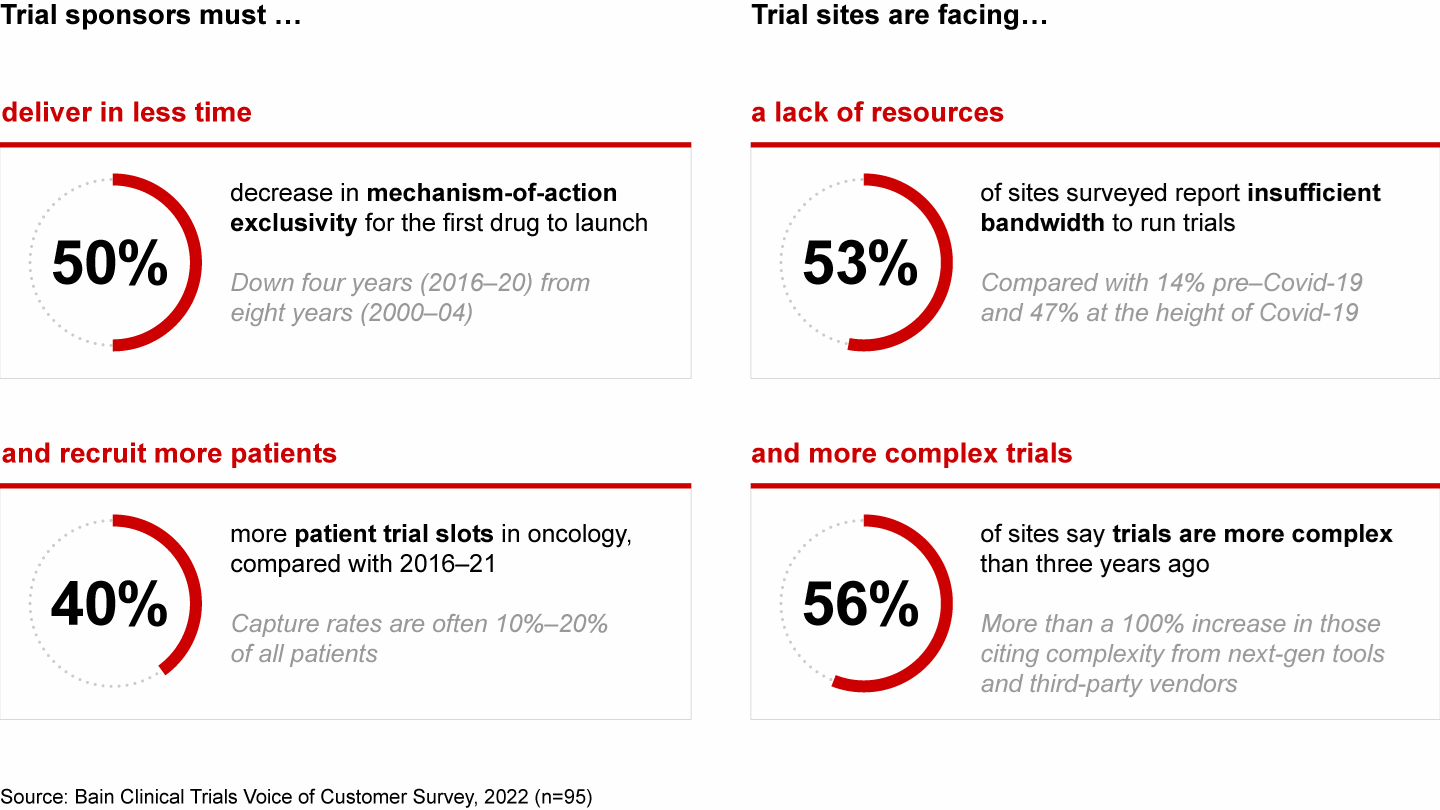
Clinical trials are facing an unsustainable confluence of challenges

Behind that complexity is a surge of immense potential. Innovation and breakthrough designations are on the rise. FDA approvals nearly doubled between 2010 and 2020, including those in critical cell and gene therapies. Pharma companies are pursuing narrower and more specific indications with increasingly complex drugs, promising relief to patients—if sites can carry out more and more complicated trials. Beyond scientific complexity, operations are progressing too, as decentralization and digital tools usher in the future of clinical trials—one of expanded patient pools and virtual interaction.
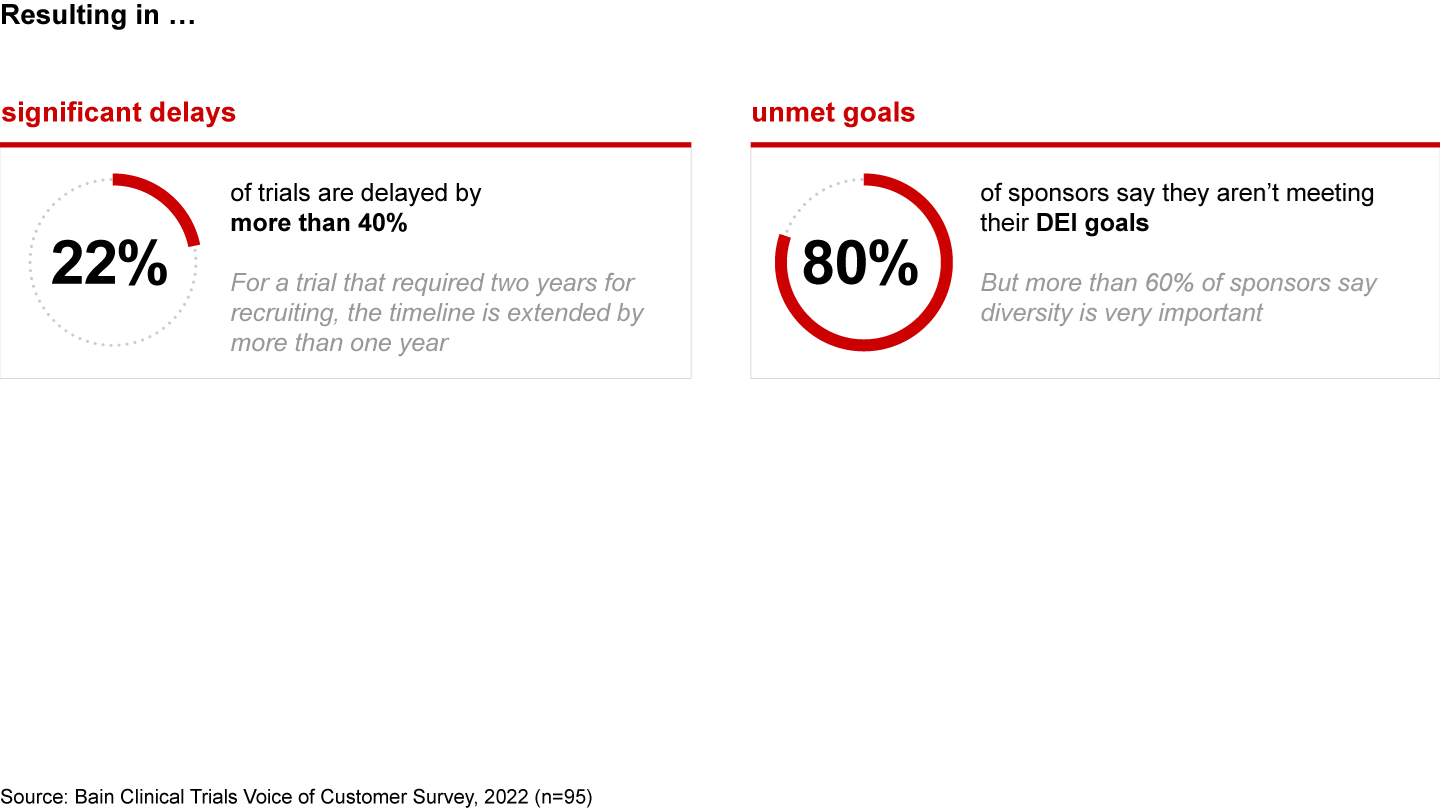
It's a vicious cycle: Rapid innovation begets complexity, and unmanaged complexity stymies innovation. As a result, more than 1 in 5 trials are delayed by more than 40% of their original timelines. Sites are being forced to make tough trade-offs and turn down trials. Pharma companies are falling short on other key targets as well. For instance, 80% of sponsors say they aren’t meeting their diversity, equity, and inclusion (DEI) goals.
The numbers cast a harsh light on the challenges that sponsors, contract research organizations (CROs), and sites have been grappling with even before the pandemic. Clinical trials are buckling under the pressure. If left unchecked, the current ecosystem will hinder the pharma industry’s ability to bring groundbreaking and necessary treatments to patients.
To unleash a wave of life-changing innovation, sponsors and CROs will need to tackle fundamental issues with both trial design and delivery, starting by developing a true understanding of site and patient needs. Our recent survey and expert interviews reveal that sponsors’ and CROs’ investments are largely misaligned with sites’ actual experiences and pain points. So long as this disillusionment remains, so will the trial delays and frustrations.
Expectations vs. reality
As the race to market picked up in recent years, sponsors and CROs have pushed to increase the number of trial sites and boost patient recruitment. They’ve invested in a patchwork of solutions for hybrid trials, such as third-party patient recruitment vendors, wearable devices, and electronic patient-reported outcomes.
These moves toward decentralization add value—but at a high cost. The lack of a comprehensive, integrated solution proliferates unbridled complexity that drags on the site and patient experience. And these investments haven’t addressed sites’ biggest concerns. While most sponsors are focused on traditional topline results, most sites are struggling with upstream challenges to starting up trials. For instance, when citing their top three concerns, 58% of sites note institutional review board approval, 44% point to the contracting and activation process, and 40% indicate accessing and managing multiple sponsor systems (see Figure 2).
Sponsors and trial sites aren’t aligned on top challenges

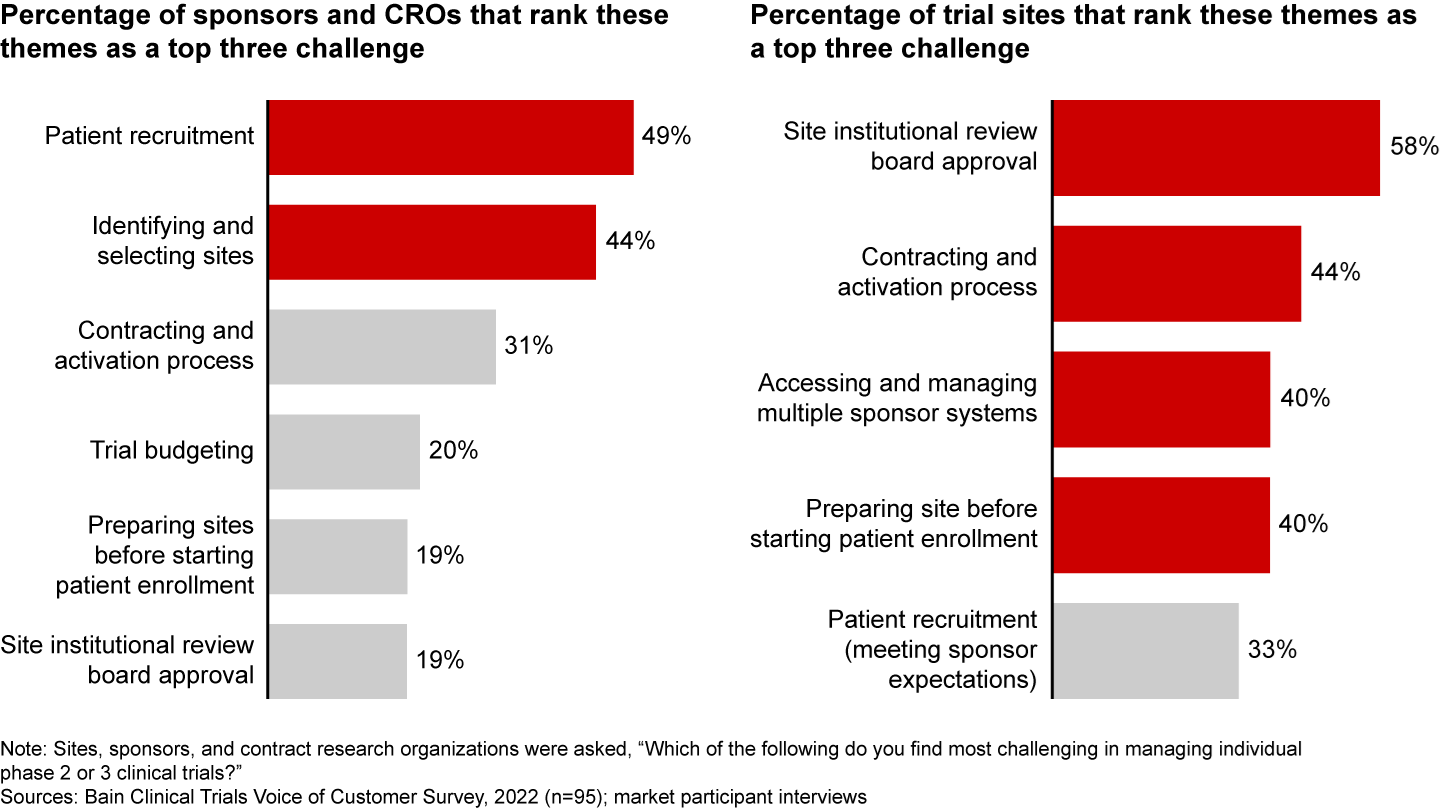
It’s evident that there’s a mismatch between what trial solutions tout and what sites truly experience throughout the clinical trials journey.
- Sponsors’ expectation: Seamless, remote site activation.
- Sites’ reality: Sites also want remote site contracting and start-up. But multiple stakeholders from the sponsor and the CRO are typically involved in activation, without a clear point of contact. Sites and CROs find themselves caught in back-and-forth emails for multiple rounds of negotiation, with no clear end in sight.
- Sponsors’ expectation: Remote training at the physician investigator’s discretion.
- Sites’ reality: Sites want rapid training, responses to questions, and issue resolution for investigators. But training material is typically dense. Information is spread across multiple portals and data sources. And with the rise of remote monitoring, it’s more difficult for investigators to engage representatives.
- Sponsors’ expectation: A steady flow of qualified patients identified with advanced analytics or recruited through outsourced support.
- Sites’ reality: Sites are interested in new digital sources to identify patients, such as automated electronic medical record review or digital marketing. But the current flow of inbound referrals can be choppy. It often starts with an overwhelming deluge of qualified and unqualified patients that takes too much time to sift through, resulting in high screening failure rates. Even when the flow is steady, there typically isn’t an integrated system to manage incoming patient referrals and track them through randomization, when needed.
- Sponsors’ expectation: Remote monitoring of patients at physician’s discretion, with greater appointment flexibility and fewer logistical burdens.
- Sites’ reality: Sites want advanced analytics capabilities, as well as passive and automated data collection, to monitor ongoing studies. But providers find themselves burdened with learning new digital monitoring tools, training patients on the tools, interacting with patients across several unintegrated platforms, and troubleshooting system issues.
- Sponsors’ expectations: Expanded site footprints that reach different cities and rural areas to advance DEI goals.
- Sites’ reality: While expanding site footprints and physician outreach is a critical endeavor, the current complexity often requires infrastructure beyond many underserved sites’ capabilities. Community sites are usually coping with less resources than larger centers. Until sponsors mitigate complexity, these efforts may have the unintended effect of hindering patient recruitment.
Sites are understandably frustrated as these pain points go unaddressed. According to our survey, the number of sites reporting complexity due to decentralization has increased 120% from three years ago. Those that note complexity related to digital tools is up 111%.
Adding third parties to the mix often seems to be doing more harm than good. Given the need for increased coordination, sites citing third-party vendors as a source of complexity has doubled. And sponsors aren’t happy with the outcome either. Only 13% say that outsourced patient recruitment support adds significant value.
The competitive advantage of customer experience
The timing of these piecemeal structural changes and injection of new digital tools has only added to the strain on sites. While sponsor and CRO staffing levels haven’t bounced back to prepandemic levels, the situation has improved since 2021. Sites, however, are reaching a breaking point due to high staff turnover and difficulty finding qualified candidates. More than half report insufficient bandwidth to support phase 2 and 3 trials, up from 14% before the pandemic and 47% at the height of Covid-19. Under the pressure of labor shortages, sites are becoming increasingly selective in which trials they take on, making difficult calls to close trials, and charging more for remote trials.
As the competition for sites escalates, the investigator experience is becoming a powerful source of advantage. Bain research shows that investigators who have a good experience with a sponsor enroll twice as many patients than those who have a neutral or negative experience.
Thus, there’s a major benefit in catering to sites’ wants and needs and building investigator loyalty—namely, faster trials. Pharma companies can’t afford to take a wait-and-see approach. And creating an industry-leading trial experience will require more than the quickly deployed, poorly integrated point solutions of the past.
Transforming trials
Future winners will take a proactive, customer-first approach to transform trials end-to-end. While overhauling the system from design to delivery is a daunting task, the current model is unsustainable, and the situation will likely worsen as issues continue to compound. But five principles can help pharma companies construct a seamless trial experience.
Instill a customer mindset and culture underpinned by collaboration. Leaders will prioritize holistic organizational change, oriented around a best-in-class patient and site experience. They’ll ensure collaboration across all functions and partners by questioning existing silos. These companies will also clearly communicate each function’s role in delivering a superior customer experience and managing complexity.
Zero-base trial design with hard trade-offs around complexity. The best trials will be fit-for-purpose, with every design element, from scientific to operational factors, determined by putting specific site and patient needs first. The key to real, lasting change is a commitment to making hard trade-offs. Leaders will push back on complexity created by nice-to-have solutions, while finding ways to mitigate customer challenges that stem from necessary scientific complexity.
Reinvent the front line. For the front line to work together as a unified team, members will need the right training, support, and tools. Leadership teams can focus on more disciplined account management, deeper site relationships, and the empowerment to solve challenges creatively. After taking these approaches with site monitors and medical affairs team members, one leading pharma company saw sustained increases in site engagement and subsequent enrollment.
Design a best-in-class site experience. Leading companies will collect routine, recurring feedback from sites and investigators. They’ll make real-time updates to integrate what they learn into the tools, processes, and technology that they deploy consistently across sites.
Invest in supporting infrastructure. Winners are getting ahead with proactive investments in performance management, from comprehensive dashboards to cross-functional customer relationship management tools for sites and investigators. For instance, one company built out end-to-end dashboards for everyone from the front line to the C-suite to monitor trial performance and make real-time adjustments in activities and resources. It also created an integrated frontline account management software used by all site-facing stakeholders on medical affairs and clinical teams to provide site-level analytics, account planning and tracking, and reporting on issue resolution.
Whether developing a bespoke platform or selecting one of the many emerging options for clinical trial services, leading companies will tailor their performance management to the needs of their organization, their sites, and their vendors. As difficult and complex as it can be, they’ll invest in unified portals and data systems that are as integrated as possible, rather than fragmented solutions. It’s critical for the tools to come together seamlessly across people and processes to achieve their full value and transform clinical trials.
To start addressing issues head on, pharma companies can launch redesign pilots for high-priority assets and programs. They’ll need to bring together the right stakeholders early in trial design—not later during trial delivery—to discuss issues, commit to trade-offs, and creatively address trial needs in the most seamless, least complex way possible. As this team tests and learns, it can scale the best processes and capabilities across the organization. This transformative approach will go beyond reducing delays to increase speed to market, build competitive advantage, and bring critical innovations to the patients that need them.